Natural History Curators
Fieldwork 2018
This year’s fieldwork was our 17th in the alpine of the northern B.C. We made collections from six mountains. The area is so vast and remote and access is difficult, thus few if any biological inventories have been undertaken in many large areas. Many peaks and lakes have no names; in fact there are no names on entire mountain massifs. I feel like we are to some degree just ‘scratching the surface’ of what is out there.
We again worked together with the insect and spider experts at the museum. And we followed our typical approach of setting up camp for 2-3 days and collecting specimens of every species we encounter, being intentional to reach as many different habitats as possible.

Typical day of hiking (dotted line) and collecting, striving to reach as many habitats as possible. Upper part of figure documents elevation changes during the day. Three of the four plants in this illustration were encountered only once, highlighting the importance of having several botanists in the field and covering as much ground as possible.
At one mountain, Mt. Whitford, we were joined by two staff and a contract photographer of the Yellowstone to Yukon Conservation Initiative https://y2y.net/about-us and their guest freelance journalist who produces pieces for both CBC and NPR. At a second mountain, south of Tumbler Ridge, we were joined by two staff members of the Tumbler Ridge Geopark, http://tumblerridgegeopark.ca/. What is a Geopark? According to their website “A UNESCO Global Geopark is an area recognized as having internationally significant geological heritage.” These groups are all interested in knowing as much as possible about the biota of these areas and we will share everything we learn with them.
As we have in the past, we contacted the local indigenous groups and informed them of our work and will provide them species lists when the identifications are complete.
We are often asked if we notice any of the effects of climate change during our fieldwork. Treeline is controlled by temperature, not elevation and is highest at the equator – where temperatures are warmer at higher elevations – and becomes lower and lower further north and south. One likely consequence of a warming planet is that forests will advance into the alpine, reducing the available habitat for tundra plants that generally require open, i.e. non-shaded habitats.

Young trees in alpine meadows at 1750 m north of Laurier Pass, Graham-Laurier Provincial Park. Note the dark skies of a storm about to dump
For a number of years I’ve noticed small trees in the alpine and of course wonder if their appearance is related to climate change as a consequence of global warming. I’ve also noticed the absence of dead trees. The absence of dead trees may mean that tree populations in the alpine are relatively young, compared to lower elevation forests where trees have been growing and dying for thousands of years.
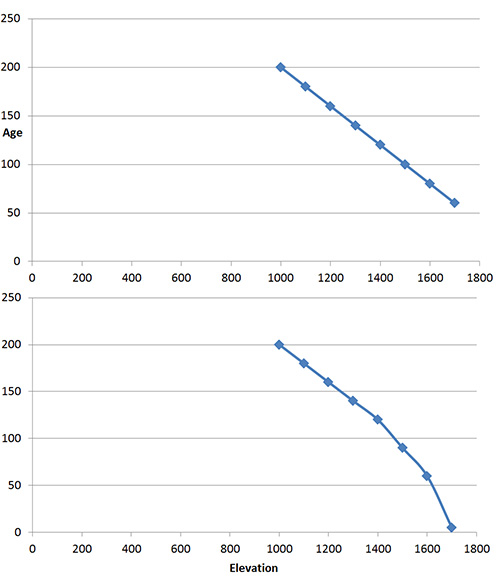
Two hypothetical graphs of tree age versus elevation curves. The upper one portrays trees that become gradually younger with elevation gain, much as one might expect if treeline was moving higher gradually. The hypothetical curve on the lower graph describes a more abrupt change, i.e. what one might expect if tree line had been stable for a relatively long period of time, slowly moving to higher elevations, then suddenly and relatively recently, young trees became established higher than before.
But a further question is this: have the young trees in the alpine arrived relatively suddenly and recently because of recent rapid increase in global temperatures, or are they gradually moving upwards due to a long term warming trend that has been taking place ever since the end of the Pleistocene, ca. 13,000 years ago? Dating trees by their growth rings could provide the answer by measuring the ages of trees along an elevation gradient from well below tree-line into the alpine. I suspect this kind of research is underway.
Every year we seem to encounter botanical surprises, either range extensions or species that we haven’t seen before. I like these kinds of discoveries because distribution patterns tell us something about the history of the landscape and when those distribution patterns are found to be different from what was previously known, the background story might change.
One notable collection this year was Dodecatheon frigidum (northern shootingstar) that we collected in northern Graham Laurier Provincial Park, about 200 km south of where it has been collected previously near the Alaska Highway. We’ve visited 8 mountains in the intervening area and have not encountered this species. What does this occurrence mean? Have we merely overlooked it in other areas or is it in fact not present for this 200 km distance?
Another interesting find was Claytonia lanceolata (western spring beauty) which I saw in the alpine for the first time. Previously I had encountered it at lower elevations in Botanie Valley north of Lytton. Indigenous people in the southern interior of BC eat the tubers either fresh or cooked. Perhaps indigenous people in the Tumbler Ridge area also eat the tubers. I haven’t had a chance to ask local people or to investigate the literature.
When is a holotype not a type specimen?
When it was never published in the first place.
The Royal BC Museum fish collection contains a specimen which had been locked securely in one of our type cabinets since the 1980s. It was designated as the holotype for a new species – Sebastes tsuyukii – there was even a manuscript noted on the specimen label (Westreim and Seeb 1989). It sounded legit – and no one checked until recently.
Jody Riley – my ever diligent volunteer – flagged this record when she was re-organising the fish collection. She checked what is in our old paper catalog, checked the electronic database, then looked to see if the actual specimen exists. When Jody hit Sebastes tsuyukii, and found no record of the species online, yet here in her hands was the jar with a big yellow tape label saying Holotype for Sebastes tsuyukii, she knew something was fishy.
Did the manuscript stall during composition, submission, or revision? Who knows.
In the end, we can take this large jar out of the cabinet designated for type specimens, Sebastes tsuyukii now is a nomen nudum (a naked name), and I can delete the species from the taxonomy in our museum database. Some database problems are easy to solve.
But this reminds me to get my fingers in gear and type the type descriptions for species I have yet to publish.
Orca Abscessed post
Nitinat (T12A) was a well known Orca along the BC coast. Born in 1982, he was a fixture along the BC coast and an active participant in the 2002 attack on a Minke Whale in Ganges Harbour, Saltspring Island. This animal – with its characteristically wavy dorsal was found dead off Cape Beale near Bamfield, September 15th, 2016. Funds weren’t available to prepare the entire skeleton, so I had to settle for the skull and jaws.
As you can imagine, the head of an orca would pop the frame of any domestic chest freezer, and it blocked the aisle of the walk-in freezer at the Pacific Biological Station in Nanaimo. It was also no small feat to fork-lift the head into the museum’s van, and then get it back out of the van and wheel it to the museum’s walk-in freezer. It also was a surreal experience driving around with an orca head in the truck. The head is heavy – and slippery – and difficult to tie down – so I drove smoothly to avoid having the head roll around behind me. Imagine explaining to an insurance company how an orca head caused you to lose control of your vehicle?
Nitinat’s head was prepared by Mike DeRoos and Michi Main – their internationally acclaimed business, Cetacea, focuses on cleaning and articulating whale skeletons. While preparing this skull for burial, they noticed that Nitinat had broken teeth. Given that I broke a molar on a frozen Reese’s Piece in a Dairy Queen Blizzard, I could imagine how Biggs Orcas could break a tooth when biting down on a sea lion or elephant seal. Large pinnipeds have dense bones.
Once the skull was cleaned, Mike and Michi found that not only were teeth broken, there also is a nickle-sized hole in the palate and many teeth were abscessed. The hole in the palate is particularly interesting. It has smooth sides and so certainly had healed before Nitinat’s death. Was it a puncture and the source of the infection that caused the distortion of the teeth? Or was it a channel for the abscess to weep into Nitinat’s mouth (not a pleasant thought regardless).
Normal teeth (left) have a long root and recurved crown, with natural wear for their ecotype – but the abscessed teeth were stunning with their broken crown and expanded root. They almost remind me of some squash varieties that are available.
One of the teeth is so swollen that it couldn’t be removed from its distorted socket.
Red lines beside the skull indicate expanded tooth sockets – perhaps age and infection combined to create this effect. The sockets for the abscessed teeth were eroded and far larger than normal sockets (in this non-mammalogist’s opinion). Erik Lambertson made a great scale bar.
Nitinat’s teeth are enough to make anyone who has had a toothache cringe, and a dentist’s eyes pop with fascination. I am just waiting for the day someone requests to see Nitinat as the focus of a pathology research paper. For now, he is a permanent addition to the Royal BC Museum collection and will soon get his official catalog number.
I don’t know if the title of this article is an accurate way to say fork-tailed lizard in German, but the Gabelschwanz-Teufel – the P-38 Lightning (the fork-tailed devil) could take a lot of punishment and still get home at the end of a sortie. A fork-tailed lizard has a parallel story – it has taken a beating and survived.
It is common to find lizards with regenerated tails or tails that are recently dropped – with their tell-tail stump. Sometimes the tip is lost, others about 90% of the tail is lost. The regrown tail segment is never as nice as the original and has different scale patterns and colouration.
This male Wall Lizard photographed by Deb Thiessen, lost its tail near the base and the regenerated tail is obvious. Its meal had a perfect tail.
I have seen fork-tailed, even trident tailed lizards in photos – I remember images like this in the books I poured over earlier in my ontogeny. Had I ever seen one in person? Not until now. During my PhD thesis work, the only fork-tails I thought about were thelodont fishes known from Early Devonian rocks of the Northwest Territories.
This July, Robert Williams, a colleague from University of Leeds in England was here working on Wall Lizards. He was trying to determine if our native Northern Alligator Lizards react in any way to the scent of the European Wall Lizard.
Live animals are not allowed at the Royal BC Museum, so Rob had to perform scent trials in my dining room. The lizards were held in containers in my kitchen – and I thank my wife for her patience.
The work helps give a frame of reference to reactions between the native Sand Lizard in the UK and introduced Wall Lizards, but you’ll have to wait to hear the results. While hunting Wall Lizards on Moss Rocks here in Victoria, Rob caught a fork-tailed specimen.
Since this was such a neat specimen I requested it be saved intact for the Royal BC Museum’s collection. Here is a photo of a fork-tailed Wall Lizard from England, but Rob had to come all the way to the Pacific coast of Canada to catch one.
Lost Soles post
In museum collections, space is critical. We can’t waste space. Every millimeter of shelving is critical. If you can arrange cabinets more efficiently, do it. Can you pack more jars in a given area? Do it. If you can make space. Do it.
I have been on a binge of deaccessioning lately. What is deaccessioning? It is the museum practice of removing accessioned/cataloged specimens from the collection. Once deaccessioned, we either send specimens to other museums where they are relevant, or give them to teaching collections or perhaps to nature centers. Only rotten specimens are destroyed. We try everything we can to re-purpose specimens before we resort to destruction.
This surfperch, Embitoca lateralis, is a rare candidate for destruction. It has been deaccessioned – someone had cranked the clamp too tight years ago and the glass at the apex of lid popped. Alcohol evaporated and by the time it was noticed, it was too late. If the fish in the jar could speak, they’d say, “There’s a fungus among us.”
Deaccessioning allows me to make space in the collection for new material. Since I am trying to keep the Royal BC Museum’s vertebrate collection focused on British Columbia, the eastern North Pacific Ocean and any adjacent territory, specimens with no relevance to this region obviously have my attention. Specimens with incomplete information (or no information), also flare my obsessive nature and are on my deaccession hit list. Space is created on a jar-by-jar basis.
Putting ‘incomplete information’ in everyday terms – if we are going to meet somewhere, you generally expect some level of detail. If I say I want to meet in Tofino in June, what would you say? Imagine now that I didn’t even give you my name – but still wanted to meet in Tofino in June. I am betting you’d put on your best Monty Python-esque King Arthur and say, “You’re a Loony.” Incomplete or missing data is a real issue.
My long suffering volunteer Jody found a jar of flatfish this weekend which had never been cataloged, but was in the collection. It was only a 125 ml jar – so not a huge waste of space. On closer inspection the fishes were identified (Parophrys vetulus, English Sole), there was a location (Tofino), and a date (June 1985).
Where was I in June 1985 – oh yea – just about to graduate from grade 12. Oh the 80s – I am listening to Duran Duran while typing this – RIO – the obvious choice with its maritime theme.
Yep, that was me in 1985.
Parophrys vetulus is a common fish here in BC, so it is likely you can catch them all around Tofino in June – but it would be nice to know which beach relinquished its sole. And when did it happen? Was it at night? Was it a full moon? On the 1st of the month, or mid month? Were they in ankle-deep water or at 10 meters depth? Open beach or a tidepool? Caught by hand or with a net? Inquiring minds may want to know. And with no collector noted in the hand-written label – I can’t even badger someone by email to jog their memory or review old field notes.
These are the lost soles Jody found. Is one of them yours?
To a museum, data is everything. If you collect and preserve a specimen, record as much as you can about the event. If you are giving me your sole, then tell me its secrets.
Lacertophagy post
I have said before that European Wall Lizards (Podarcis muralis) will eat smaller conspecifics – there are a few photos online from elsewhere on Earth – but until now I didn’t have solid evidence of lacertophagy (lizard eating) here on Vancouver Island.
However, this last week, Deb Thiessen took a few videos of a Wall Lizard eating a yearling Wall Lizard on her property just north of Victoria. These are really good videos and clearly show that Wall Lizards can stuff down a huge meal.
https://www.facebook.com/deb.thiessen.9/videos/10214455524975688/
In this first video the smaller Wall Lizard is already dead, and I suspect that the larger lizard killed it. Looks like another lizard had thoughts of stealing the meal. Sure looks like breathing is an issue while stuffing down so large a meal. Snakes solve the problem of eating and breathing by pushing their trachaea (windpipe) out of the mouth so that food does not block air flow.
https://www.facebook.com/deb.thiessen.9/videos/10214455487814759/
The victor looks like a male, and in the second video you can see how quickly it disposes of the tail rather than having that part of the meal hanging out of its mouth for a few days.
Almost all of the victor’s own tail had been lost some time ago. You can always see where its original tail ended and the re-growth takes over – the new tail is never as neatly patterned.
Be glad Wall Lizards aren’t the same size as Varanus prisca, otherwise we’d be on the menu.
Science is Serious post
Or if you are an astronomer, then your science is Sirius. If you are a geologist, then your science is pretty gneiss. Don’t take science for granite.
I have been tracking Wall Lizards now for a while – and I am sure my wife will say lizard tracking has become an obsession – a serious obsession. I look at rock walls as we drive around town. I look for lizards on our weekend hikes. I watch for lacertids when I walk our daughter too and from school. Science is serious.
I have been watching the range expansion of two nicely segregated populations of lizards in Victoria – one population is about 0.63 km SSW from our house west of Hillside Mall, and the other is about 0.24 km north of us near Doncaster School – not that I have measured.
Each year I walk the perimeter of these populations to get an idea how fast lizards disperse in urban environments – again – this is serious science. Stop laughing. I can hear you laughing. Rolling your eyes does not help.
Wall Lizards seem to spread 40 to 100 meters – and it is the young ones that do the dispersing. Why? They race off to new habitat to avoid the cannibalistic tendencies of their parents. Parents with a 40 year old trekkie in the basement may want to consider this option as an incentive to get kids to move out.
Young lizards head for the relative safety of boring lawns – garden areas with lots of structure are occupied by hungry adults. Homeowners sometimes claim their lawn is crawling with young lizards in August – when all the summer’s eggs have hatched. In contrast, adults are relatively sedentary – once they find good sunny, rocky (complex) territory, they tend to move very little from year to year.
Now imagine my surprise when I walked up my driveway last night (May 23rd, 2018) and heard the characteristic rustling sound of a lizard in our food forest (yes, the lawn is gone and we have a food forest – the entire front garden is devoted to plants we can eat, and plants that attract bees to pollinate the plants with edible bits – but I digress). The lizard I found is at least 0.24 km from the nearest known population of lizards in my neighbourhood, and is an adult – with a perfect tail too – must have lived a charmed life free of bird and domestic cat attacks. Did this adult go walkabout? I doubt it.
The new colonist in the food forest at UF1510 (yes, as sci-fi nuts we gave our place a code name Urban Farm1510)…
Furthermore, the lizards nearest to my house are not brightly coloured – in fact they are kind of drab as far as Wall Lizards go. But our new lizard is gorgeous – more like ones from Triangle Mountain or farther north on the Saanich Peninsula.
This male is from Durrance Road – far more colourful than the ones near Doncaster School or Hillside Mall.
Is this a case of seriously good science prank? Was this a drive-by lizarding? Did a neighbour just buy some new garden supplies and a stow-away lizard emerged to find utopia in our food forest? I may never know.
My daughter has named the lizard Zoom. I guess he is there to stay.
Here’s a link to a new paper by: Luke R Halpin, Jeffrey A Seminoff, and myself.
Source: Northwestern Naturalist, 99(1):73-75.
Published By: Society for Northwestern Vertebrate Biology
This new paper provides the first photographs of a Loggerhead Sea Turtle (Caretta caretta) from west of Vancouver Island. The species has been spotted in the region before and as far north as Alaska, but until now, there were no photographs or specimens as solid evidence.
While the photos in this paper are black and white – the original photographs by Luke Halpin are color and van be viewed upon request. PDFs also are available – just send me an email.
British Columbia is now within the range of 4 species of marine turtle. This Loggerhead survived into February of 2015 because of the unusually warm water in the eastern North Pacific Ocean (the Warm Water Blob), whereas Green Sea Turtles (Chelonia mydas) and Olive Ridley Sea Turtles (Lepidochelys olivacea) wash up dead (or near dead) in early winter. Unfortunately, the fate of the Loggerhead from 2015 is unknown.
Nanaimo Invasion post
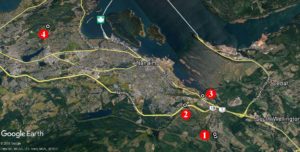
Years ago after coming off parental leave, I found a series of photographs of Wall Lizards and a Google Earth image of a road intersection marked to show locations for a lizard colony. Quick search in Google Earth showed that this colony was in Nanaimo. I fired off a fast blog article to generate interest and get people looking for Wall Lizards.
It worked. Reports came in.
Jump forward a few years and now that street (Flagstone – site 1) is crawling with lizards according to eyewitnesses. But we now also have another site (2) along the Nanaimo Parkway near Douglas Avenue and Tenth Street. Oh wait, there’s also a third site (3) in the Chase River Estuary Park, and as of this weekend, there’s another (4) – way north of the rest along Arrowsmith Road. The report of the lizards in the Arrowsmith Road area was accompanied by video – there was no doubt as to the identification of those lizards – and that was a big jump from previous known occurrences.
Two other records – one along Enfer Road near Quennell Lake, and along Leask Road south of Nanaimo have yet to be verified with specimens, photographs or video.
There you go Nanaimo, the invasion has picked up pace. Keep your eyes peeled for lizards with a green tint to their scales, minute scales on their back, and generally more delicate proportions than the native Alligator Lizard.
Look at this post to help identify any lizards in your neighborhood.
If you find suspected Wall Lizards – email me at: ghanke@royalbcmuseum.bc.ca
If you find a lizard that is not a Western Skink, Northern Alligator Lizard, or European Wall Lizard – I definitely want to know about it.
Please record the date and street address (or prominent landmark) to pin down exactly where the lizard was seen. A photo would be really helpful to confirm the lizard’s identification. Happy hunting.
The Special Issue of Aquatic Invasions on “Transoceanic Dispersal of Marine Life from Japan to North America and the Hawaiian Islands as a Result of the Japanese Earthquake and Tsunami of 2011” has been published as Volume 13, Issue 1, pages 1-186 (totalling more than 220 pages with supplementary files).
The Special Issue includes the 14 papers (all Open Access) by 39 researchers, including Dr. Henry Choong, Curator, Invertebrate Zoology.
Funding support was provided by the Ministry of the Environment (MOE) of the Government of Japan, through the North Pacific Marine Science Organization (PICES), which made this Special Issue possible.
The Introduction to the Special Issue provides vignette summaries of examples of notable Japanese Tsunami Marine Debris objects, and also details contributions to the knowledge of Japanese and North Pacific marine biota as a result of JTMD research. These contributions include new species, new species records for Japan, and a rediscovered species (last documented in 1929). A final summary table in the Introduction provides examples of molecular genetic contributions to our understanding of JTMD biodiversity.
The JTMD project, which commenced in 2012, and which is now entering its 6th year of research, as we continue to monitor for the potential arrival of living species, 7 years after the tragic disaster of March 11, 2011.
Calder, D.R., Choong, H.H.C., Carlton, J.T., Chapman, J.W., Miller, J.A., and Geller, J. 2014.
Abstract
Fourteen species of hydroids, including two anthoathecates and 12 leptothecates, are reported from the west coast of North America on debris from the tsunami that struck Japan on 11 March 2011. Six species were found on a dock that stranded at Agate Beach, Newport, Oregon, five from a boat at Gleneden Beach, Oregon, four from a dock in Olympic National Park, Washington, and two from a boat in Grays Harbor, Washington. Obelia griffini Calkins, 1899, the most frequently encountered species, was collected on three of the four derelict substrates. Eight of the species are known to be amphi-Pacific in distribution. Of the rest, at least five (S tylactaria s p . ; Eutima japonica Uchida, 1925; Orthopyxis platycarpa Bale, 1914; Sertularella sp.; Plumularia sp.) are not previously known from the west coast of North America. Hydroids of E. japonica occurred as commensals in the mantle cavity of the mussel Mytilus galloprovincialis Lamarck, 1819. Obelia griffini, O. gracilis Calkins, 1899 (not its secondary homonym Laomedea gracilis Dana, 1846) and O. surcularis Calkins, 1899 are taken to be conspecific. Of the three simultaneous synonyms, precedence is assigned to the name O. griffini under the Principle of the First Reviser in zoological nomenclature. The species is sometimes regarded as identical with O. dichotoma (Linnaeus, 1758).
Henry H. C. Choong* and Dale R. Calder
Invertebrate Zoology Section, Department of Natural History, Royal Ontario Museum,100 Queen’s Park, Toronto, Ontario, Canada, M5S 2C6
*Corresponding author
Abstract
The leptothecate hydroid Sertularella mutsuensis Stechow, 1931 is reported on debris from the 2011 Japanese tsunami that came ashore on 5 June 2012 at Agate Beach north of Newport, Oregon. Its discovery on a barnacle (Semibalanus cariosus) from a derelict floating dock originating at Misawa, Honshu, confirms the capability of successful transoceanic dispersal for this species. We compare our specimens to Stechow’s syntype material of S. mutsuensis in collections at the Zoologische Staatssammlung München, and designate a lectotype and paralectotype of the species.
Key words: Leptothecata; hydroid; lectotype; transoceanic dispersal; anthropogenic debris; Oregon coast
The Power of 1 post
It is always satisfying to update taxonomy in the museum’s database or find and correct mistakes. This week I spent some time sorting out details on Royal BC Museum specimens of California Yellowtail (Seriola dorsalis) and Great Amberjack (Seriola lalandi). Turns out that since these fishes were collected, Seriola dorsalis has been sunk, and all our fishes are Seriola lalandi (as noted by Gillespie 1993). This carangid fish is known to move into our waters in warmer years.
While reviewing what we knew about the first BC specimen (979-11312) it became obvious that the Royal BC Museum’s database was missing some information for that fish. Fortunately, this information was easily updated – the original report was published in the Royal BC Museum’s extinct periodical Syesis (see Nagtegaal and Farlinger 1980).
Drawing of Seriola dorsalis – oops lalandi (979-11312) by K. Uldall-Ekman.
In fixing that record, I noticed that some online sources had given incorrect coordinates for this fish. Contrast the capture location of 54°35’N, 131°00’W in Caamaño Passage as reported by Nagtegaal and Farlinger (1980), with online sources which state the fish was caught at 54°35’N, 31°00’W. That missing 1 in the reported longitude determines which ocean is linked this fish.
The takeaway message? Always check the original paper rather than relying on internet sources. Precise data is everything – and in the words of a well known scoundrel: “Without precise calculations we could fly right through a star, or bounce too close to a supernova and that’d end your trip real quick, wouldn’t it.” Or in this case, you’d be landing southwest of Iceland to look for Great Amberjack.
References:
Gillespie, G.F. 1993. An Updated List of the Fishes of British Columbia, and Those of Interest in Adjacent Waters, with Numeric Code Designation.Canadian Technical Report of Fisheries and Aquatic Sciences 1918. 116 p.
Nagtegaal, D.A. and S.P. Farlinger. 1981. First record of two fishes, Seriola dorsalis and Medialuna californiensis, from waters off British Columbia. Syesis 13:206 –207.
I’ve received a steady series of emails this year detailing European Wall Lizard locations here on Vancouver Island, and it’s now April and wall lizards certainly are active. However, an email arrived April 11th which gave me a WTH (What The Herp) moment. The email contained a beautifully focused photo of a new turtle for BC. Then it occurred to me that I’d lost count of how many turtle species have been dumped here – unwanted pets that outlived the interest of their owners.
I really like when people send me photos of things they think are unusual – and this week’s email was no exception. We know that Red-eared Sliders (Trachemys scripta elegans), Yellowbelly Sliders (Trachemys scripta2), and a Map Turtle (Graptemys sp.) have been dumped in Goodacre Lake, and Red-eared Sliders into Fountain Pond, but this new turtle photographed by Deb Thiessen (see below) certainly was not just an odd coloured slider, nor was it another map turtle. As an aside, I haven’t had a chance to catch the Map Turtle in Beacon Hill Park to get a good look at it, but I have seen it at a distance, and ID’ed it based on photos from Darren Copley and James Miskelly. It looks like a False Map Turtle (Graptemys pseudogeographica). I think that’ll be a summer goal, to get good photos of that turtle to be sure which species it represents.
A Peninsula Cooter (Pseudemys peninsularis) from Fountain Lake, Beacon Hill Park, Victoria, BC. Photograph by Deb Thiessen, retired CRD Parks naturalist.
As you can see from Deb Thiessen’s photograph, this new turtle has a large shell for the size of the head, and the stripes on the neck are crisp, and bold yellow offset by black. The bold markings to me suggested Peninsula Cooter (Pseudemys peninsularis). The short claws on its forelimb indicate it is female. Males would have claws double the length of those in the photo. This animal is way outside its normal range – Peninsula Cooters are from Florida.
This animal brings our list of pet turtles to 10 species abandoned in BC ponds and lakes – that we know of. Here is the list I have of turtles that have been found in BC – way out of their native range – and (shockingly) it parallels species available in the pet trade here in BC.
Trachemys scripta (Pond Slider – both T. s. elegans and T. s. scripta)
Pseudemys peninsularis (Peninsula Cooter)
Pseudemys concinna (River Cooter)
Chrysemys picta marginata (Midland Painted Turtle, possibly also Southern Painted Turtles, C. p. dorsalis)
Graptemys pseudogeographica (False Map Turtle)
Emys orbicularis (European Pond Terrapin – always did like the word Terrapin – a bit of nostalgia from my British roots)
Chinemys reevsi (Reeve’s Turtle)
Malaclemys terrapin (Diamondback Terrapin)
Apalone spinifera (Spiny Softshell Turtle)
Chelydra serpentina (Common Snapping Turtle)
Fortunately most turtles are dumped one at a time and do not reproduce. Sadly though, I can’t say the same for the Red-eared Sliders – they now can reproduce successfully here in British Columbia (I have two pets from the first successful clutch found on the south coast of BC, ca. January 11, 2015). Red-eared Sliders now are common in artificial and natural ponds and in lakes here in southwestern British Columbia – and until recently, we were sure that each adult represented an abandoned pet (or maybe the occasional escapee). Now males are finding females. Females are finding decent nesting locations. And eggs are surviving to hatch.
Knowing that sliders can breed here, I stopped to check whether sliders and cooters can hybridize, and it has been suggested to be possible – but no solid proof. And since it is better to be safe than sorry… Does anyone know how to neuter a Cooter?
This time of year, my garden is one big mudslide. Sunny days with a blue horizon are not that common here on Vancouver Island in winter – but when they occur, we certainly enjoy them. So do our slim little European Wall Lizards.
This January and February I collected lizards which were active when the air temperatures were between 5° to 7°C. As a survivor of the Canadian prairies, collecting lizards in winter seems about as strange as an empty room in a museum collection.
I found lizards along Derby Road in my neighborhood, on Moss Rocks, at Gardenworks Nursery in the Blenkinsop Valley – winter lizard activity is nothing new here on Vancouver Island.
Lizards were found in south-facing locations with full sun exposure and when caught, were very warm to the touch. It is obvious that they are effective solar collectors and can elevate their body temperatures well above that of the chilly air – even when it is a bit windy. It is not uncommon to see lizards only exposing their head for a while, then the rest of the body. Perhaps this is a low-risk way to warm blood via blood vessels in the throat before they venture out and deal with intruding conspecifics. I haven’t seen any wall lizards feeding in winter – but that doesn’t mean they don’t. I’ll have to examine museum specimens to see what’s in the stomachs of winter-caught lizards.
An adult European Wall Lizard caught on Derby Road in Victoria, February 26th, 2018.
As of this February, the Royal BC Museum collection has 30 lots of European Wall Lizard specimens representing surface activity for each month of the year. Some people collect trading cards to get a complete set, I collect lizards to get one per month. Wall Lizards are active in winter as far north as Denman Island, and given that range, probably could extend further north of Campbell River in areas with a warm microclimate.
The collection of lizards for each season put a song from 1971 into my head – so I reworded the chorus a bit…
Winter, spring, summer or fall,
All they have to do is crawl,
And I’ll be there, yes I will,
Their spread has to end.
Tristan A. McKnight & Robert A. Cannings
Abstract: Stackelberginia cerberus sp. nov. (Diptera: Asilidae) is described from the Amargosa desert (USA: Nevada) and compared to related taxa. This is the first record of the genus in the Western Hemisphere; other species live in the deserts of central Asia. Stackelberginia Lehr is proposed as the sister taxon to Lasiopogon Loew in the subfamily Stichopogoninae based on morphological characters and a Bayesian species tree estimated from one mitochondrial (COI) and three nuclear protein-coding loci (AATS, PEPCK, wingless). Stackelberginia has the medially divided epandrium and rotated hypopygium of Lasiopogon, but the facial gibbosity is flat, macrosetae of thorax, head, and legs are unusually long, and phenology peaks in late autumn.
Key words: Stichopogoninae, robber fly, assassin fly, species tree, molecular, Palearctic
Joel. F. Gibson
Abstract: The thick-headed flies (Diptera: Conopidae) are rarely observed parasitoids. Confirmed hosts include many species of bees and wasps. Often collected from flowers, conopids may serve as either pollinators or pollinator predators. The last detailed checklist of the Conopidae of British Columbia was published in 1959. An updated checklist for British Columbia, the Yukon, and Alaska is presented based on over 1,000 specimens and specimen records. Geographical distribution, using an ecoprovince approach, is documented for each of 26 species in the region. Host, plant association, and hilltopping behavioural records based on past literature and new observations are also included. An identification key to all species recorded is included.
Key words: parasitoid, biogeography, plant associations, host associations, Nearctic
Colin J. Curry, Joel F. Gibson, Shadi Shokralla, Mehrdad Hajibabaei, and Donald J. Baird
Abstract: We reviewed the availability of cytochrome c oxidase subunit I (COI) sequences for 2534 North American freshwater invertebrate genera in public databases (GenBank and Barcode of Life Data Systems) and assessed representation of genera commonly encountered in the Canadian Aquatic Biomonitoring Network (CABIN) database. COI sequence records were available for 61.2% of North American genera and 72.4% of Insecta genera in public databases. Mollusca (73.9%) and Nematoda (15.4%) were the best and worst represented groups, respectively. In CABIN, 85.4% of genera had COI sequence records, and 95.2% of genera occurring in >1% of samples were represented. Genera absent from CABIN tended to be uncommon or members of groups not routinely used for biomonitoring purposes. On average, 94.1% of genera in well-identified samples had associated sequence data. To leverage the full potential of genomics approaches, we must expand DNA-barcode reference libraries for poorly described components of freshwater food webs. Some genera appear to be well represented (e.g., Eukiefferiella), but deposited sequences represent few sampling localities or few species and lead to underestimation of sequence diversity at the genus level and reduced confidence in identifications. Public COI libraries are sufficiently populated to permit routine application of genomics tools in biomonitoring, and ongoing quality assurance/quality control should include re-evaluation as new COI reference sequences are added or taxonomic hierarchies change. Next, we must understand whether and how established biomonitoring approaches can capitalize on high-throughput sequencing tools. Biomonitoring approaches that use genomics data to facilitate structural and functional assessments are fertile ground for future investigation and will benefit from continued improvement of publicly available sequence libraries.
Key words: COI, invertebrates, biomonitoring, high-throughput sequencing, DNA metabarcoding, identification,
genus, Biomonitoring 2.0
Robert A. Cannings & Russell V. Pym
Archilestes californicus McLachlan (California Spreadwing) is a large damselfly native to western North America, ranging from Washington and Idaho south to New Mexico, Arizona and California and, in Mexico, to Sonora and Baja California Sur (Paulson 2011; Westfall and May 2006). This note records the species for the first time in Canada—from three sites in the southern Okanagan Valley, British Columbia (BC; Figure 1).
Russell Pym saw several males and females at a small, shallow, artificial pond at the end of an artificial stream near the entrance to the Liquidity Winery at 4720 Allendale Road, Okanagan Falls, BC (49.32553°N, 119.54993°W). He observed them from 13:00 to 14:00 PDT on 26 September 2016; one male was photographed (Figure 2). From 16:30 to 17:00 PDT the same day, he recorded a female in knee-high grass, three to four metres from the shore of a dugout pond across the road from Walnut Beach Resort, 4200 Lakeshore Drive, Osoyoos, BC (49.01825°N, 119.43580°W). Cattail (Typha latifolia) and willows (Salix spp.) lined the pond margins.
You and Yew post
Plants make molecules that chemists could never imagine. Chemical poisons that deter herbivores are advantageous for organisms that can’t move. For humans, depending on the dosage, these molecules can be either poisonous or medicinal. The small shrub circled here is Pacific Yew. In 1962 it was discovered that its tissues, especially the bark, contain ‘taxol’ effective in treating cancer. It took many kg of bark (in 1993 34,000 kg were harvested in BC) for a single dose – and killing the tree. Now the drug is synthesized in a lab using precursors from the needles. The seed is surrounded by red, fleshy tissue called an ‘aril’, sometimes incorrectly called a ‘berry’.

The small shrub circled here is Pacific Yew.

The seed is surrounded by red, fleshy tissue called an ‘aril’, sometimes incorrectly called a ‘berry’.
Swordfishes in BC post
In an earlier post I mentioned that Luke Halpin was out surveying marine mammals and birds from the deck of the CCGS John P. Tully, and spotted something totally different west of Brooks Peninsula. The fish was estimated at 3.5-4 meters in length, and was cruising against the current just below the surface.
But until the paper announcing his find was accepted by a scientific journal, I didn’t want to spill the beans and say what he had found. His research paper (Halpin et al. 2018) will be published in the spring issue of the Northwestern Naturalist.
Photo by Luke Halpin, September 5th, 2017
This picture says it all – there is no debating what this fish is – only one species that fits the bill. Swordfish are known north to the southern Kuril Islands in the western Pacific, but Luke’s find is the northern-most record for the species in the eastern Pacific and is conclusive evidence of this species right along our coast.
A Google Earth image showing where the Swordfishes from 2017 and 1983 were found relative to Vancouver Island.
A previous record from 1983 (see Sloan 1984, and Peden and Jamieson 1988) was from just inside of our exclusive economic zone (EEZ) and barely qualified as a BC fish. The 1983 specimen was caught as by-catch at 47°36’N, 131°03’W, during an experimental fishery survey by the M/V Tomi Maru. The rostrum and tail were preserved in the Royal BC Museum’s fish collection (RBCM 983-1730-001). I am guessing the edible bits in between were cut into steaks, and ended up on someone’s dinner table. At least Luke’s Swordfish was left alone and for all we know, is happily cruising south to slightly warmer water.
References:
Halpin, L.R., M. Galbraith, and K.H. Morgan. 2018. The First Swordfish (Xiphias gladius) Recorded in Coastal British Columbia. Northwestern Naturalist, 99(1): XX-XX. (pages not set)
Peden, A.E., and G.S. Jamieson. 1988. New distributional records of marine fishes off Washington, British Columbia and Alaska. Canadian Field-Naturalist, 102(3), 491-494.
Sloan, N.A. 1984. Canadian-Japanese Experiental Fishery for Oceanic Squid off British Columbia, Summer 1983. Canadian Industry Report of Fisheries and Aquatic Sciences No. 152: pp. 42.
We Three Kings post
Keep your eyes peeled for deep-sea fishes while strolling along our shores. In the last month, three King-of-the-Salmon (Trachipterus altivelis) have washed up in the Salish Sea. Two were found in September (21st and 26th) in the Oak Bay area, Victoria. One of these was still swimming when found. A third was found October 3rd in Hood Canal, in Puget Sound. The first Oak Bay specimen will be preserved for the Shaw Centre for the Salish Sea in Sidney, the second was not recovered, and the third will be preserved in the Burke Museum’s collection. The Royal BC museum has 18 Trachipterus specimens, with several of these from the Salish Sea area.
The King-of-the-Salmon from Hood Channel, photographed by Randi Jones.
Is this species new to the region? No. The species ranges from Alaska to Chile, and knowledge of this species pre-dates European arrival on this coast. Is this trio of King-of-the-Salmon a case of post-spawn mortality? A sign of change in our oceans? We don’t know. Actually, when you look at the diversity of marine fishes off our coast, there is a lot of basic biology that we don’t know. We also get Longnose Lancetfishes (Alepisaurus ferox) washing up from time to time, although it has been a few years since I have heard report of a Lancetfish in the Victoria region.
King-of-the-Salmon swim by passing a sine wave down their dorsal fin – they can get a fair bit of speed just by doing that. They can also reverse using the same fin flutter. They slowly turn by putting a curve in the body. However, in the first few seconds of the linked video you can see that they also swim in a more typical fishy way (using eel-like body oscillation) when they need a burst of speed or a really quick turn. If you’d like to see this form of locomotion in person – you can see it in a pet shop. Knife fishes use the same basic locomotion method – except they use their anal fin rather than the dorsal.
Close up of the head of the King-of-the-Salmon showing the premaxillary (red) and maxillary (green) bones extended, photographed by Randi Jones.
Note also in the video that the fish has a very short face compared to the Hood Channel specimen photographed onshore. As with many fishes, the jaws of the King-of-the-Salmon are protrusible – the premaxillary and maxillary bones swing out to create a tube – the gill chamber dilates, and water rushes into the mouth along with the prey. The same sort of suction pump mechanism is used by a wide variety of fishes – from tiny seahorses to giant groupers. Once the prey item is inside the fish’s mouth, the mouth closes, water is released through the gills and the prey is swallowed. The entire sequence is lightning fast – even in pipefishes and seahorses – blink and you miss it. In some fishes, the process is even audible – you can hear a snapping sound when seahorses slurp up crustaceans (and fishes). You can’t hear the same snapping sound when larger fishes engulf their prey, but it is no less dramatic an effect.
In 2014, a Louvar and a Finescale Triggerfish were found in BC – a double-header of interesting southern fishes in our waters. But wait… it looks like 2017 is also a double-header for cool coastal fish.
This summer of 2017 (and in 2016), Basking Sharks were sighted here in BC. I think every Basking Shark is newsworthy given that they were nearly eliminated here in an ill-conceived plot to protect BC fisheries (see Wallace and Gisborne 2006 for that sad story). This year’s Basking Sharks were found in Caamano Sound in July, and near the Delwood Seamounts in August. Was it one roving shark? Or two? Are there others?
This September however, Luke Halpin was out surveying marine birds from the deck of the CCGS John P. Tully, and spotted something totally different west of Brooks Peninsula. The fish is estimated at 3.5-4 meters in length, and was cruising against the current just below the surface.
We are really fortunate that it was sunny and seas were so calm – because his picture leaves no doubt as to the fish’s identification. The best part about the story is that the fish is still out there. Don’t get me wrong, I’d have loved to have the fish as a specimen for the museum’s collection – but then again, it would require a custom vat – three to four meter fishes don’t fit in jars.
This species is known north to the southern Kuril Islands in the western Pacific, but Luke’s find is the northern-most record for the species in the eastern Pacific and is conclusive evidence of this species as a new addition to our coastal fish fauna. Which species did he find? You’ll have to wait until he publishes his observations in a scientific research paper. Consider this a trailer – a teaser – there’s a big fish out there – it is cool… and I am jealous. I would love to see this fish alive.
100 Meter Dash post
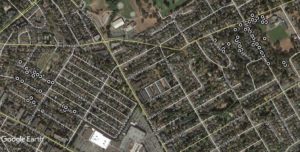
The Doncaster population of the European Wall Lizard probably is 6 years old based on conversations I have had with home owners. In the Google Earth image – the white dots are known locations – the green dots are new locations for 2017.
How do I know these are new? Homeowners specifically said they had no lizards in 2016 – but they certainly do now. That’s the power of local knowledge and citizen science. The green dots along Oak Crest Drive were newly reported in the spring of 2017, with at least three adult lizards now known on the property. The two green dots along Cedar Avenue to the northeast are based on sightings of at least three young lizards – probably lizards that hatched this year and got well-clear of their parent’s territory. Cannibalism is a good emigration motivation.
Based on where lizards were known in 2016, these 2017 records represent range extensions from 20 to 100 meters. Compared to their body size, that’s pretty decent dispersal given that adult lizards only grow to 21 cm (those fortunate enough to have a perfect tail), and in many cases, the dispersing lizards are young-of-the-year at 8 or so centimeters in total length.
If younglings continue to bolt at this rate and make a bee-line south, I will have lizards in my garden in 2 years. More realistically, it will be another 3 years before we see them along our raised beds or in our greenhouse – not that I’m counting.
We now have 21 orca specimens at the Royal BC Museum—the latest to arrive was T-171, a 6.07 meter female Biggs Orca which was found near Prince Rupert, October 19th, 2013. She had pinniped skulls, vibrissae (whiskers) and partially digested bones in her gut but was emaciated. Why was she emaciated?
During the necropsy, researchers discovered that T-171 had mid-cervical to lumbar vertebrae with severe overgrowth of the neural arches and lateral processes (noted as spondylosis in the necropsy) – the overgrowth looks roughly like popcorn or cauliflower – and had the effect of interlocking some vertebrae. This likely explains her emaciated state. Was she able to hunt? Was she supported by her relatives?
The skull of T-171 (ventral (palatal) view [left], right side [center], and dorsal view [right]) awaiting its catalog number and final place in the Royal BC Museum collection.
Comparison of T-171’s vertebra (left) with overgrowth of bone vs. the normal vertebra of another Biggs Orca (12844) (right). The two vertebrae are not from the exact same position along the spine, but the difference between the two is still shocking.
Many of T-171’s vertebral centra are eroded and porous – not like those of a healthy animal (12844).
The overgrowth of the neural arches pinched the spinal chord of T-171; compare to a neural arch of 12844 (right). The vertebral malformation must have limited this animal’s mobility. It is hard not to anthropomorphize and imagine the discomfort due to this deformation.
T-171 originally was prepared for exhibit at the Royal Ontario Museum, but they wanted a clean articulated skeleton for exhibit. In contrast, we were interested in T-171 because of its skeletal malformation. To make a short story long, we came to an agreement with the ROM to transfer T-171 to the Royal BC Museum, and since, the ROM has acquired L95 (Nigel), a 20 year old southern resident who was found near Esperanza Inlet, March 30th, 2016.
Which Orca is next? In most cases we have no clue – it is not like we hunt orca just to add them to the collection. And we don’t usually have a production line of specimens in preparation. New specimens are acquired when a body washes up, and we make a snap-decision to cover the cost of specimen recovery and preparation. However, September 15, 2016, T-12A (Nitinat) was found off Cape Beale and towed to Banfield. I was contacted September 16th to see if the Royal BC Museum was interested (obviously that was a YES), and now his massive skull is being prepared. Once degreased, Nitinat’s skull will be added to the Royal BC Museum collection – sometime in 2018 – and made available for scientific research.
As a kid I collected many things – from reptiles and amphibians to model airplanes to Star Wars cards – and now look where I am. I dress in black and white as a Stormtrooper with the 501st legion and collect black and white delphinids – Killer Whales – for the Royal BC Museum. Life sure takes you to unexpected destinations.
A little while back I was musing over a spot on my Wall Lizard map that shows a large expanse east of Highway 17 between Cordova Bay Road to Mt Newton Cross Road that appears to be Wall-Lizard-free turf. Wall Lizards are crawling everywhere just the other side of the highway on Tanner Ridge. Either no one has reported lizards from this area – and it seems unlikely given how many reports I receive each year, or lizards have not been able to cross HWY 17.
Cedar Hill Road in the Southeast Cedar Hill area also seems to be a decent barrier even though it is not a particularly busy road. Lizards have been in that area for about 6 years(as of 2016) and have crossed Derby Road without a problem – but not Cedar Hill Road. Cedar Hill may be just busy enough to limit the survival of adventurous lizards.
It seems interesting that a lizard as fast as the Wall Lizard could not cross – but then again – why would they? Young ones disperse to avoid cannibalism, but perhaps the noise, vibration and sight of passing vehicles is enough to dissuade all but the most suicidal of lizards.
I recently tripped across an article detailing road crossing behaviour in snakes (Andrews and Gibbons 2005). In their study, smaller snakes seemed to avoid crossing roads, whereas larger snakes have no problem with the concept. I wonder if the same is true for Wall Lizards? Interestingly, all snake species they studied crossed perpendicular to the road’s length – an adaptive behaviour minimizing distance and time on the tarmac. Some species froze in place when a car passed – that is maladaptive – and significantly increased an animal’s exposure to vulcanized rubber.
I have not seen Wall Lizards crossing a street – but would be interesting to see if they too cross perpendicular to the curb, and whether they blast across or dart and pause – unintentionally increasing their risk of catastrophic z-axis reduction.
Andrews, K.M., and Gibbons, J.W. 2005. How to Highways Influence Snake Movement? Behavioural Responses to Roads and Vehicles. Copeia 2005(4): 772-782.
Another lizard arrived in BC last week. We can add Brown Anole (Anolis sagrei) to our list of accidental imports – but this certainly is not the first one to have arrived by accident in BC. Many lizards travel the globe as stow-aways. This one travelled here in its egg along with a Snake Plant (also known as the Mother-in-Law’s Tongue). Sansevieria are popular houseplants – Snake Plants are easy to keep and look neat. My wife bought one for our living room – no lizards in our plant though.
Where was the plant from? Who knows. This plant could have come from anywhere. Brown Anole’s have invaded Florida, and southern parts of Georgia, Louisiana, Mississippi, and Alabama. They also have invaded Hawai’i, southern Texas and southern California along with their relative the Green Anole (Anolis carolinensis). The Green Anole is native to the south-eastern United States, and in their native range, Green Anoles may be forced out of their usual habitat by their exotic relatives. Brown Anoles are native to Cuba and the Bahamas.
Even if it got loose, this anole would not survive our winter. It was no real threat to our environment or fauna, but does show that the transport of exotic species is ridiculously easy – an egg in the soil in a plant pot. This time we are fortunate. Only one egg was present. Anoles are light-weight arboreal lizards which lay one egg at a time, and they are not parthenogenetic. Anole eggs develop in alternate ovaries at about a two week interval – if I remember correctly. This ensures the female lizard is not excessively encumbered, and for us it meant that only one egg likely was present in the pot (or any other pot at the home hardware store).
Brown Anole eggs are a bit bigger than a Tic-Tac candy, so no wonder they are overlooked – they also are buried a centimeter or so in the soil – so they’d be out of sight. As long as the soil was not disturbed, was warm and moist – but not too wet, and the egg was not rolled, the developing embryo would survive transport.
I wonder where this lizard’s brothers and sisters ended up? They could be anywhere. Since the lizard travelled here in an egg, I vote we name it Mork. Na-Nu Na-Nu.
Just tripped across this fish while sorting out odd records in the RBCM fish database.
999-00114-001 – unidentified fish – Family Triglidae (Searobins, Gurnards)
Well, it turns out to be Prionotus stephanophrys – a Lumptail Searobin – and a new family, genus and species for BC. Three other triglid species (two of them are Prionotus species) are known to stray into Atlantic Canada.
This one was caught in 1998 on La Perouse Bank, it was added to the RBCM collection in 1999, and sat there ever since. No one had taken a second look at this specimen – until today. It was completely new to our system and as such, I had to add the genus and species to our database’s taxonomic tree.
Until now, its northern record was off the mouth of the Columbia River – this new(ly rediscovered) record extends this family north about 260 km in the eastern North Pacific Ocean.
The lower three pectoral rays of this fish are almost like fingers – it probably walks along the bottom like other triglids – the walking mechanism makes me think of face-hugger Alien larvae.
I took these photos of Royal BC Museum lizard specimens with my iPhone 4 through the eyepiece of the old dissecting microscope in my lab. Then sent the photos via two emails to office thanks to WiFi – and to think – this is the “low-tech” way of doing things these days. Low-tech – sending files through the air from a hand held device… I have to laugh how technology has changed since I was a kid with my first pet lizards. The nerd in me can’t help but hear James Earl Jones’ voice – “Several transmissions were beamed to your inbox. I want to know what happened to the scans they sent you.”
In earlier blogs I have mentioned scale differences between BC lizards – so I thought I may as well take close-up shots to clearly show the differences. Under a dissecting microscope (diss-secting, not die-secting), you can easily see the shape of the bead-like back scales of the European Wall Lizard (Podarcis muralis). It’s like a microscopic cobblestone pavement. Each scale is about the diameter of a standard sewing pin.
European Wall Lizard (2112)
The larger back scales of the Northern Alligator Lizard (Elgaria coerulescens) are painfully obvious, and each scale has its own raised keel. The keel gives each scale an angular appearance.
Northern Alligator Lizard (1358)
The Pygmy Short-horned lizard (Phrynosoma douglasii) has a really complex squamation with tiny granular scales interspersed between clusters of larger keeled scales. The larger scales are raised into spires above the general scale-scape (the lizard equivalent of landscape).
Pygmy Short-horned Lizard (323)
Western Skinks (Plestiodon skiltonianus) by contrast are painfully even and smooth – yawn. It’s a good thing they have speed-stripes and a bright blue tail to make them stand out in a crowd.
Western Skink (1964)
Western Fence Lizards (Sceloporus occidentalis) have scales each with a trailing spine – characteristic of all Sceloporus species. Some, like the Crevice Spiny Lizard in the United States have really robust spines on their scales, others like the Sagebrush Lizard have tiny spines. Cordylids in Africa take spiny scales to a whole new level.
Western Fence Lizard (705)
Sorry, I forgot a scale bar in the photos, but the images were fairly close to the same magnification.
Geraldine A. Allen1 & Laurie J. McCormick1 & Johanna R. Jantzen1 & Kendrick L. Marr 2 & Becky N. Brown3
Abstract: Phragmites australis (common reed) is a widespread perennial grass of wetland habitats, with cryptic native and introduced subspecies in North America. We determined the relative abundance of the subspecies and the distributions of plastid DNA haplotypes throughout British Columbia, Canada, at the northwestern distribution limit of common reed in North America. Of 203 specimens assigned to subspecies using molecular markers, we identified only 9 plants as the introduced ssp. australis; all remaining samples were the native ssp. americanus. The two subspecies co-occurred at only one locality. We identified four native haplotypes (one widespread in British Columbia and three others more localized) and two introduced haplotypes. Using plants of known haplotype, we assessed the utility of different morphological traits and trait combinations for distinguishing native and introduced subspecies in this geographic region. No single morphological trait was diagnostic, but principal components analysis and identification indices based on combinations of traits consistently separated the native and introduced subspecies in our sample. Two- or three-trait combinations of ligule length, lemma length and stem anthocyanic coloration gave the best separation. These indices could reduce the need for confirmation of the introduced subspecies using molecular tools, facilitating efforts to monitor and control this invasive plant.
You’d think that sharks and rays would be pretty well known along our coast. Did you know that two Hammerhead Sharks have been found off Vancouver Island? Even a Tiger Shark has strayed north to Alaska. Did it swim along the BC coast, or did it take a more direct route from Hawai’i? We’ll never know. However, in 2016 a new shark was added to our fish fauna – the Pacific Angel Shark (Squatina californica) – based on a clear photograph by Mark Cantwell and his detailed description of the dive location.
We have known since 1931 that Angel Sharks ranged north to Seattle, and there is a single record from Alaska. The specimen label for this 35 cm Alaskan female had been lost (Evermann and Goldsborough 1907) and we cannot pin down its collection location with certainty. Until now, we had no Angel Shark records for British Columbia – but it was only a matter of time.
On the 30th of April, 2016, a single adult Angel Shark was sighted by a diver off Clover Point right here in Victoria. The shark’s gender cannot be determined from the photograph since claspers, if present, are not visible. The Angel Shark was found in about 12 meters of water, about 30 meters off the point. The diver estimated the shark’s length at about 1.1 to 1.2 meters in length. The specimen was not collected, but it would have made a fantastic museum specimen.
King and Surry (2016) published the discovery of this shark in BC in a recent issue of the Canadian Field-Naturalist. While this now is not breaking news – in fact it is a year late – people may still want the primary reference to our latest elasmobranch.
PDFs are available here [as a new paper, King and Surry (2016) is available by subscription to The Canadian Field-Naturalist or by contacting the primary author]:
Belted Kingfishers (Megaceryle alcyon) usually take fishes – why else would they be called kingfishers. They sometimes take crustaceans and frogs, and I’d be shocked if they turn their beaks up at big juicy insects. However, mammal predation is quite a dietary shift. Apparently no one explained the meaning of “fisher” to a kingfisher in the southwestern Yukon.
This female obviously read its species description. Looks like she caught a young goldfish. (From: https://en.wikipedia.org/wiki/Belted_kingfisher#/media/File:Belted_Kingfisher_with_prey.jpg)
A paper came out in a recent issue of the Canadian Field-Naturalist (see Jung 2016) detailing the capture of a Western Water Shrew (Sorex navigator) by a Belted Kingfisher. That would make a decent meal and a real energetic boost for the Kingfisher. Jung (2016) mentioned that Belted Kingfishers have been known to take Eastern Water Shrews (Sorex albibarbis), and he (Jung 2013) also reported on a kingfisher trying to subdue a Spotted Bat (Euderma maculatum).
From: https://upload.wikimedia.org/wikipedia/commons/8/8f/Belted_Kingfisher.jpg
Imagine if kingfishers changed tactics to regularly prey on other small animals? Their ecology could converge on that of butcher birds (shrikes). What’s next? Lizards and snakes?(Yes, shrikes impale their prey on thorns (or barbed wire) to age a bit).
Sure glad kingfishers aren’t the size of a Banshee or Leonopteryx from Avatar, or we’d all be at risk when swimming.
Keep your eyes on the sky. And as for that specific Water Shrew, all you can say is: “Hair today, gone tomorrow.”
PDFs are available here:
Was this an odd title? Actually I think the song went,
“On top of spaghetti… all covered with cheese,
I lost my poor meat ball… When somebody sneezed.
It rolled off the table… and onto the floor.
And then my poor meat ball… rolled out of the door.
Wow that was a dredged from deep cephalic crevices…
Anyway, I got a tip from Purnima Govindarajulu, my herpetological counterpart in the Ministry of Environment that she’d seen a European Wall Lizard on Mount Tolmie here in southern Saanich. Given how fast and far Wall Lizards are spreading, it was only a matter of time before they colonized this rock. This pocket of lizards will form another expanding sub-population – pretty-much midway between the single lizard I saw at the University of Victoria and the lizards near Doncaster School.
This morning (April 27th) was nice and sunny, and I hiked up to the summit after dropping my daughter at daycare. What did I find first? A Northern Alligator Lizard. That made me very happy – I don’t see those everyday and this lizard was more than patient with the iphone-wielding twit who wanted its picture.
Then less than 2 meters away were the Wall Lizards – five of them. A meter or so along the road, another Wall Lizard. Up along the southeast corner of the reservoir – another large male Wall Lizard.
Yep, looks like they have found a solid toe-hold in this region. Cedar Hill X Road may make a decent barrier to northward dispersal (not that Wall Lizards aren’t north of there anyway) – but they will easily spread southeast and southwest into gardens adjacent to the park. Note the small scales and green colour on this Wall Lizard’s back, compared with the larger coppery scales on the Alligator Lizard (above).
Keep your eyes on rock gardens, rock walls, woody debris, and any bedrock with decent cracks for shelter. The photo below shows just how slender the Wall Lizards are – this one with an intact tail is the largest lizard I have caught to date (21.2 cm total length). After checking the RBCM’s herps database, I see that the only months where I haven’t caught Wall Lizards are January and February – too bad that this spring was consistently cold and wet. I have missed my chance to get a full year’s worth of lizards in 2017.
Somnolent Sculpins post
Yesterday I worked with Chris O’Connor from our Learning Department – we took some children on a tidepool tour. The main point was to chat about museum collections and things we record or measure when we are out sampling. We didn’t go crazy catching fishes, only taking 3 Tidepool Sculpins (Oligocottus maculosus) in the end. But we talked about our role as museum researchers, and why we take more than 1 specimen (if possible) to get an account of variation within and between species.
You can see slight differences between these fishes – even an injury – just like the subtle, or not so subtle differences we see in each other.
The three fishes will be added to the Royal BC Museum’s ichthyology collection, but before that, they are fixed in 10% Formaldehyde. Researchers used to drop fishes directly into Formaldehyde – many fishes died horrible deaths. When I accidentally get Formaldehyde in a cut – it stings intensely – I couldn’t imagine being dunked directly into that chemical.
Today we are more humane, and give fishes an overdose of anaesthetic before immersion in Formaldehyde. They are dead before they are fixed, and are preserved with a relaxed posture. The primary anaesthetic I use is 2-Phenoxy-Ethanol, but it is hard to get without ordering from a chemical supply company, and the chemical is a suspected carcinogen. I still have about 500 ml of the stuff – so I will use up what I have. Do I really want to buy more? Maybe not.
Do we have safer options? Yes, Clove Oil is a good anaesthetic if mixed as an emulsion in a small volume of 99% Ethanol. But you have to carry a jug of 99% Ethanol everywhere you go – that may not go over well at a Police check-stop. The up-side to this chemical mix is that you smell spicy at the end of the day if you accidentally spill some on yourself.
People have tried Alka-Seltzer tablets. They fizz and release CO2, which knocks-out fishes – but the process is slow and some fishes (those like catfish that gulp air to survive in low oxygen conditions) are resistant and survive way too long in a stressful condition.
A few months ago I tried using Oragel (20% Benzocaine) on European Wall Lizards – colleagues had found it worked well on amphibians. They put Oragel along the spine of an amphibian and it soaks into the skin; I give lizards an oral dose. It renders bullfrogs and wall lizards unresponsive in 20 seconds to a minute. Oragel seems to be a convenient anaesthetic for these invasive herpetiles.
Yesterday, I told the tidepool group that we’d be performing an experiment – I tried Oragel for the first time on the 3 sculpins we caught. As I hoped – less than 20 seconds and the fishes were out cold. 2-Phenoxy-Ethanol takes about the same time on similar sized fishes.
The beauty of Oragel is that it is readily available, and if you run out, you can stop by the nearest pharmacy. It also is safe – we use it on sore teeth or gums. Perfect – it works fast on specimens and is safe for the researcher.
Perhaps someone needs to do a larger scientific study to see how effective over-the-counter Oragel is on larger fishes. Maybe this is an effective over-the-counter tool for preserving new museum specimens.
A Big Shark in BC post
A specimen with no data is not worth keeping. A specimen with vague data is not worth keeping either. The Royal BC Museum’s ichthyology collection contains a vertebral centrum with cartilaginous remnants of its respective haemal arch and neural arch from a shark that washed up November 5th, 1975 (only a few months after Jaws was released in cinemas). It was cataloged as 976-00052-001 in the fish collection (with a variant of the catalog number listed as a previous number ~ B.C.P.M. #97652). Our electronic database only had a collection date for this centrum (no location, no collector).
Flip to our original paper catalog, and we find that there is indeed a collection location: Ahousaht Village, Flores Island – but this never got translated to our electronic database. The paper catalog states that the shark washed up on a beach – but there was no latitude and longitude provided for the record beyond 49°N, 125°W. If you plot the western-most limit of 125°W, it is nowhere near Flores Island – so the location is questionable. Ahousaht Village’s nearest beach is at about 49°16’N, 126°03’W.
Worse yet, the vertebral centrum indicates that this was a big shark – we don’t have a lot of big sharks here…
Great White Shark (Carcharodon carcharias) reaches 6 meters
Pacific Sleeper Shark (Somniosus pacificus) reaches 5-6 meters
Basking Shark (Cetorhinus maximus) reaches at least 9 meters
The shark centrum in the Royal BC Museum collection is about 7.3 cm in diameter – it spans most of the palm of my hand. This must have come from a decent-sized shark. Was it a small Basking Shark? A large Great White? A large Sleeper Shark? It’s not ‘reptilian’ so we can rule out Cadborosaurus (whew). Hang on, Cadborosaurus’ so-called “type specimen” was a photograph of a digested basking shark – Hmmm…
It is a shame no one bothered to take a skin sample – the scales may have been diagnostic. What about teeth? A sample of teeth – even one tooth – would have been enough to identify this fish. Sadly though, nothing remains other than this centrum and a bit of cartilage. It was fixed in formaldehyde and stored in isopropanol – so I think we can forget sending a chunk to Guelph for DNA barcoding. DNA barcoding wasn’t a thing back in 1975, so tissue samples were not preserved for future analysis.
If no one in Ahousaht has a photo of this shark on the beach, or some teeth stashed away, all I have to say is , “Sorry Charlie, the Royal BC Museum wants specimens with good data.”
A Winter so Harsh post
This winter has been cold here in Victoria – relatively speaking. We have had lots of rain, several rounds of snow – and I even had to shovel my driveway and sidewalk. Actually I have had to shovel several times this winter. The rest of the country is not all that sympathetic to the wintery-woes of its Pacific Islanders.
One odd feature of Victoria is that Anna’s Hummingbirds are present year-round – because people feed them. Without artificial feeding stations, they likely would migrate south in autumn with the Rufus Hummingbird and return each spring. It still strikes me as strange to see a hummingbird in winter – given that I moved here from Winnipeg.
In my neighbour’s yard there is Holly bush that is a regular nesting site for our resident male Anna’s Hummingbird – the spot must be coveted because the prickly leaves are a great deterrent to would-be nest thieves.
This nest from 2005 was near the junction of Government Street and Niagra Street in James Bay – also in a Holly bush.
Our hummingbird – yes we are possessive even though we don’t feed hummingbirds in winter – is a regular visitor to our veggie garden and flowers in summer. It stayed this winter even though it was snowy and cold. Someone nearby must have a hummingbird feeder.
Not all Anna’s Hummingbirds were so lucky this year. Today I received a nest containing two feathered nestlings which were snuggled together in their soft little lichen-cup nest. This is certainly an early nesting attempt – they are known to nest from February to August, but nesting this early in the spring is a big risk.
The fate of the female is a mystery (males don’t raise their young). Did she hit a window? Run short of food and die? Did a free-range domestic cat get her? These two nestlings were in a sheltered spot alongside a house here in Victoria, but without a parent, they didn’t last long. Natural selection can be as cold as this winter.
In 2006 I spent a month at sea on the CCGS W.E. Ricker, collecting hundreds of deep sea fishes during a Tanner Crab Survey. Most fishes were identified the traditional way using anatomical features, but we didn’t have an extensive library on board, so many ‘field’ identifications were wrong. Such is life on the high seas when you are rushed to process samples.
Several snailfishes and of course the poorly known Flabby Whalefishes were only identified to genus. One snailfish with its distinctive pelvic girdle resembling a pair of bat’s wings – was simply labeled as “Batwing.” It was a few years later while sorting out some of the samples, that I tripped across a paper by David Stein (1978) describing our “Batwing” species in detail – Osteodiscus cascadiae. Keep in mind that the last comprehensive book on BC fishes – Pacific Fishes of Canada – was published in 1973… I was 6 years old. Pacific Fishes of Canada needs an update – it is woefully out of date.
This week I have been cataloging the last of the fishes caught on the 2006 Tanner Crab Survey – Screech – I know what you are thinking. A decade has passed since these fishes were caught. I am not a slacker – well, some would argue that – but there are many reasons why I am only now sorting and cataloging the last of the Tanner Crab specimens. Forgive me if progress is slow.
Many of the specimens we collected in 2006 had a small plug of tissue removed for DNA Barcoding. Three specimens (DNA barcode field tags from left to right, G5036, INV792, and 0738-Bo2), from Queen Charlotte Sound and west of the northern end of Vancouver Island were identified as Careproctus canus. If this is correct, they are the first for British Columbia.
The same can be said for specimens (barcode field tags from left to right, R5826 and G5026), both from Queen Charlotte Sound which were identified as Careproctus attenuatus. If correct, they are the first of their kind for BC, and both species C. canus and C. attenuatus, are way-south of their known ranges in the Aleutian Islands. We also caught one other snailfish identified as Paraliparis melanorhabdus (15943) – if correct it is the first specimen for the RBCM, but not the first for BC.
When I got down to the last few unidentified fishes to catalog in the RBCM database, I found that they had tags from the DNA Barcoding project. Obviously I looked up the molecular identification, but I have to wonder whether a genetic sequence was used to identify these new snailfishes, or whether the DNA barcoding team used our field identifications. We certainly do not carry an exhaustive library at sea, and we do our best to identify fishes with what we have at our finger-tips while the decks are heaving and rolling. Since I don’t trust my own eye regarding snailfishes – these noteworthy records need to be verified – and I think I’ll send them to a snailfish expert that I know just south of the border.
However, two specimens of Gyrinomimus (lovingly known as Flabby Whalefish) were identified as G. grahami (barcode tags, left to right INV0718 and R5828), and both were from west of the northern end of Vancouver Island. They don’t look much better in person. We left these specimens identified to genus because we had no literature for Flabby Whalefishes on board. As a result, I know the species-level identification did not come from me – and had to be based on molecular information. YAY, Gyrinomimus grahami (15942, 15935) is new to BC.
These interesting records alone justify the time taken to collect and send DNA samples to Guelph for the barcoding project. I may not be a gene-jockey, but if the identifications of these fishes are correct, we will rack up another three new species for BC, boost our knowledge of biodiversity, finally have two of our whalefish specimens o-fish-ally identified. Now to compare the newly identified whalefish specimens to the other 10 jar-loads of specimens to see if we have one or more species in our collection.
Thanks all you DNA barcoders – particularly Dirk Steinke who was out with us in 2006 – couldn’t have done this without you.
Abstract
In Canada, there are no native catfish west of the continental divide and until recently, the list of extant exotic catfishes in British Columbia only included introduced Black Bullhead (Ameiurus melas) and Brown Bullhead (Ameiurus nebulosus). We report that a single Yellow Bullhead (Ameiurus natalis) was collected from Silvermere Lake in the Lower Fraser River drainage. This represents the first record of the Yellow Bullhead in western Canada, and its introduction likely was accidental with a shipment of Largemouth Bass (Micropterus salmoides) rather than dispersal from Washington. Warm, eutrophic, weedy habitat in the Fraser Delta provides ample habitat for Yellow Bullheads and other exotic fishes. A Blue-eyed Panaque (Panaque suttonorum), a loricariid catfish found in 1995 in Shawnigan Lake, Vancouver Island, probably represents a single, illegally released aquarium fish, as does a large Silver Pacu (Piaractus cf. P. brachypomus), which was found in Green Lake on Vancouver Island in 2004.
ABSTRACT
Polymerolepis whitei Karatajūtė-Talimaa, 1968 was described based on isolated polyodontode scales recovered from the Ukraine, and originally was thought to be heterostracan (Agnatha). Additional scales with neck canals were described years later, and as a result, P. whitei was reclassified as a bradyodont holocephalan because it had scales similar to those of Listracanthus Newberry & Worthen, 1870. Until now, no articulated body fossils were known, and so the classification of this taxon has remained uncertain and based only on the original author’s opinion. New specimens of P. whitei from the Mackenzie Mountains, Northwest Territories, Canada, show articulated scale patches from the head, with the best specimen showing part of an anal fin, caudal peduncle, and caudal fin. This new material confirms that the original account of scale variation was accurate, but also that P. whitei possesses an anal fin spine, a feature that, until recently, was thought to be a synapomorphy of acanthodian fishes among Palaeozoic fishes. Several primitive chondrichthyans (Obtusacanthus Hanke & Wilson, 2004; Lupopsyroides Hanke & Wilson, 2004; Kathemacanthus Gagnier & Wilson, 1996; Seretolepis Karatajūtė-Talimaa, 1968; Doliodus Traquair, 1893; Antarctilamna Young, 1982, and also problematic taxa such as Gyracanthides Woodward, 1902, and now Polymerolepis Karatajūtė-Talimaa, 1968), are known from articulated remains and show a fin-spine complement like that of acanthodian fishes. They also have placoid scales or polyodontode scales that grew by areal rather than superpositional accretion. These taxa blur the distinction that exists in historic literature between acanthodians and early chondrichthyans.
ABSTRACT
New anatomical details are described for the acanthodian Lupopsyrus pygmaeus Bernacsek & Dineley, 1977, based on newly prepared, nearly complete body fossils from the MOTH locality, Northwest Territories, Canada. New interpretations of previously known structures are provided, while the head, tail, and sensory lines of L. pygmaeus are described for the first time. The pectoral girdle of L. pygmaeus shows no evidence of pinnal and lorical plates as mentioned in the original species description. Instead, the dermal elements of the pectoral region appear to comprise a single pair of prepectoral spines which rest on transversely oriented procoracoids, and large, shallowly inserted, ornamented pectoral fin spines which contact both the procoracoids and scapulocoracoids. The scales of L. pygmaeus lack growth zones and mineralized basal tissue, and superficially resemble scales of thelodonts or monodontode placoid scales of early chondrichthyans, and not the typical scales of acanthodians. However, L. pygmaeus possesses perichondrally-ossified pork-chop shaped scapulocoracoids, a series of hyoidean gill plates, and scale growth that originates near the caudal peduncle; these features suggest a relationship to acanthodians. Prior to this study, both authors conducted separate cladistic analyses which resulted in differing tree positions for L. pygmaeus and its relationships within the Acanthodii. However, both analyses did agree that there is no evidence allying L. pygmaeus to the traditional “climatiid” acanthodians contrary to previous historical classifications.
Abstract
Mid- to Late Palaeozoic sharks and holocephalans display a wide range of armour, with bodies that range from sleek, pelagic forms to slow-swimming, chimaeroids or ray-like bottom dwellers. Despite this Late Palaeozoic diversity, there still is an expectation that early chondrichthyans will be anatomically like later species. Recent discoveries from eastern Canada (Doliodus problematicus), and several heavily spined fishes from the MOTH locality in the Northwest Territories, including Kathemacanthus and Seretolepis, described here, challenge this expectation. These fishes show scale and endoskeletal features thought to be characteristic of chondrichthyans, yet they have paired fin spines, anal fin spines, and in some cases rows of prepectoral and prepelvic spines as would be expected from primitive acanthodians. Kathemacanthus and Seretolepis do not fit neatly within the cur- rent taxonomy, demonstrating that previous distinctions between acanthodians and chondrichthyans, including scale-based criteria, fail to account for the diversity being discovered in the fossil record.
Abstract:
Two new acanthodian taxa are described. The ischnacanthid Xylacanthus kenstewarti is based on large, dentigerous jaws, and Granulacanthus joenelsoni is based on isolated spines. The isolated remains of these species are similar in that they both possess pustulose denticles or tubercles, either on the mesial ridge (X. kenstewarti) or on the fin spines (G. joenelsoni). Jaws of X. kenstewarti are similar in size to those of Xylacanthusminutus, Ischnacanthus kingi, and I. wickhami and smaller than those of X. grandis. The jaws of X. kenstewarti are most similar to those of X. minutus, but are distinguished from this and other ischnacanthid species by a tapering patch of pustulose denticles that is widest midway along the jaw, mesial denticles that are simple blisterlike structures, the monocuspid, striated primary teeth that are subcircular in cross section, and a posterodorsal process that is enlarged. The spines of G. joenelsoni have distinctive tubercular ornamentation. Tubercles, or nodular ornaments on fin spines, are characteristic of primitive acanthodians, but the slender shape of the spines, the low number of spine ribs, and the fine striations posterior to the main ribs of each spine suggest that G. joenelsoni is a relatively advanced acanthodian. Xylacanthus kenstewarti and G. joenelsoni are from the Silurian (Wenlock or Ludlow) of the southern Mackenzie Mountains. Xylacanthus kenstewarti represents the earliest representative of the genus, the earliest unequivocal remains of a gnathostome from the Mackenzie Mountains, and extends the known geographical range of the genus from the Mackenzie Mountains east to Spitsbergen.
Abstract
An acanthodian, Tetanopsyrus lindoei gen. et sp. nov., is described. All specimens are from Lochkovian of northwestern Canada. The body is covered with unornamented, flat scales, with two finely noded dorsal spines, finely noded anal, pelvic and pectoral spines, a high scapulocoracoid, and toothless jawbones with large, flat, crushing surfaces. Tetanopsyrus lacks pectoral dermal plates and intermediate pre-pelvic fin spines. Tetanopsyrus is classified in the new family Tetanopsyridae, and possible relationships of the family to diplacanthids are discussed.
Abstract
Specimens of two new fish species were collected from the Lower Devonian ichthyofauna of the Mackenzie Mountains, Northwest Territories, Canada. These two species are interesting in that they have monodontode scales, lack teeth, and have an unossified axial, visceral, and appendicular endoskeleton. These characteristics have been suggested to be primitive for jawed fishes. However, the new taxa have combinations of median and paired fin spines which are similar to those of acanthodian fishes. The new taxa show no obvious characteristics to suggest relationship to any particular group of acanthodians, and for the moment, we will not try to determine their relationships, but to use them as outgroups in an analyses of relationships within the class Acanthodii. Our cladistic analysis results suggest that climatiiform fishes are basal relative to acanthodiform and ischnacanthiform taxa. However, in contrast to previously published analyses, the order Climatiiformes appears paraphyletic relative to the other two acanthodian orders. Lupopsyrus pygmaeus is placed as the basal-most acanthodian species, Brochoadmones milesi, Euthacanthus macnicoli, and diplacanthids are relatively derived “climatiiform” fishes, and the heavily armored condition in Climatius reticulatus and Brachyacanthus scutiger appears as a uniquely derived state and not primitive for all acanthodians. In addition, Cassidiceps vermiculatus and Paucicanthus vanelsti seem to be related to acanthodiform fishes based on fin spine structures. Cassidiceps vermiculatus originally was placed with climatiiform fishes in the original description. Given our character coding, we identified several primitive characteristics which were retained in relatively derived acanthodian taxa.
Abstract
A mesacanthid acanthodian, Promesacanthus eppleri n. gen., n. sp., is described based on specimens collected from the Lower Devonian (Lochkovian) Manon-the-Hill locality of the Mackenzie Mountains, Northwest Territories, Canada. The head and body resemble that of other mesacanthids, but unlike all other acanthodiforms, this new taxon has a small prepectoral spine anterior to the pectoral fin spine. This new mesacanthid also possesses ornamented, blade-like hyoidean gill covers, enlarged lobate head scales, fin spines with ribs and fine striations, a scapulocoracoid with a triangular coracoid portion and a dorsal blade which is elliptical in cross section, procoracoids that articulate with a rounded fossa on the anteromedial face of the scapulocoracoids, and jaws which articulate at a simple, single joint. Mesacanthids are thought to be basal among acanthodiforms and are grouped based on a phenetic argument and their shared retention of features which likely are primitive for acanthodiforms (most notably, enlarged head scales, blade-like hyoidean gill covers, and a single pair of prepelvic spines). Based on overall similarity, P. eppleri n. gen., n. sp. appears most similar to Mesacanthus mitchelli, but the relationships of P. eppleri n. gen., n. sp. within the Mesacanthidae have yet to be determined with a cladistic analysis.
Abstract
The acanthodian Paucicanthus vanelsti gen. et sp. nov. is described from six body fossils from Lower Devonian (Lochkovian) rocks of the southern Mackenzie Mountains, Northwest Territories, Canada. This new species is unique among acanthodians in that it lacks both pectoral and pelvic fin-spines. In the absence of fin-spines, the leading edges of the pectoral and pelvic fins are reinforced by enlarged scales. The anatomy of the acanthodiform Traquairichthys pygmaeus is similar to P. vanelsti in that both lack pelvic fin-spines, although T. pygmaeus also lacks pelvic fins. Similarly, the acanthodian Yealepis douglasi lacks both paired and median fin-spines, and its anatomy resembles that of P. vanelsti based only on the loss of paired fin-spines. The lack of paired and (or) median fin-spines in these three taxa contrasts with the widely held view that acanthodian fins all were preceded by spines. The anatomy of P. vanelsti also is similar to that of the acanthodian Brochoadmones milesi in that both have a completely unossified endoskeleton, slightly elevated pectoral fins, and deep, compressed bodies. The median fin-spines of P. vanelsti have an anterior leading edge rib followed by a field of fine striations. This striated ornamentation coupled with few leading edge ribs also is seen on fin-spines of Cassidiceps vermiculatus and primitive acanthodiform acanthodians (e.g., Mesacanthus and Lodeacanthus species). I tentatively suggest that this fin-spine ornament indicates relationship between P. vanelsti, acanthodiform acanthodians, and C. vermiculatus. However, a cladistic analysis is required to test whether or not the characteristics such as fin-spine loss, unossified endoskeleton, elevated pectoral fins, deep compressed bodies, and (or) median fin-spine ornamentation are synapomorphies within the Acanthodii or evolved convergently within the class.
Abstract
New anatomical details are described for the acanthodian Brochoadmones milesi based on nearly complete body fossils from Lochkovian rocks at MOTH, Mackenzie Mountains, Northwest Territories, Canada. The body and caudal peduncle are deep, and a prominent nuchal hump is present before the dorsal fin origin. The caudal fin is correspondingly deep and ventrally, the caudal fin lies close to and is partly joined to the slender anal fin. A delicate pectoral fin trails the flattened pectoral-fin spine where previously known specimens showed only a fin spine resembling a bivalve shell. Seen for the first time in any vertebrate, each of the six pairs of prepelvic spines supports a small, scale-covered finlet. Both prepelvic spines and scalecovered finlets increase in size posteriorly. The series of paired prepelvic finlets originates ventral to the branchial chamber and anteroventral to the pectoral fin, and extends posteriorly as far as the pelvic fins. The scales of the body and fins are thin and flat, without obvious evidence of ossified basal tissue or entry point for vascular tissue. The main lateral-line canal passes dorsal to the branchial chamber and terminates at the trailing edge of the caudal fin web. Lateral-line scales are thicker than body scales and show concentric growth zones. Scales from the dorsal midline of the caudal fin are also thicker, showing few superpositional growth zones in the mesodentine of the crown together with what appears to be cellular basal tissue. The structure and position of the pectoral spine and fin, the extremely thin body scales, the slender anal fin, and the prepelvic finlets are all unique and appear to be autapomorphic features compared to those of other acanthodians. Brochoadmones milesi is derived relative to other fishes traditionally classified in the Climatiiformes. Kathemacanthus rosulentus is removed from the Brochoadmonoidei, leaving only B. milesi in a monotypic suborder.
Abstract
An undescribed genus and species of pachyosteomorph arthrodire, Squamatognathus steeprockensis gen. et sp. nov., from the Middle Devonian (Eifelian) Elm Point Formation in Manitoba is described. It was found in the LaFarge Quarry at Steep Rock, Manitoba, and is represented by the anterior portion of a large, right inferognathal with a large terminal cusp, similar to inferognathals of the family Dinichthyidae. It has unique sculpture on the lingual surface not reported from any other dinichthyid arthrodire.
Abstract
Fish remains from the Middle Devonian (Late Eifelian) were found in three limestone quarries in the Elm Point and Winnipegosis formations, near Lake Manitoba, south-central Manitoba. The arthrodire material represents a taxon previously unknown from Manitoba. Eastmanosteus lundarensis sp. nov., is described based on an articulated, nearly complete cranial roof and incomplete cranial roof, a suborbital plate and thoracic shield fragments. E. lundarensis is the oldest representative of the genus, and is the first record of the genus in Canada. E. lundarensis is most similar to the other North American and European Eastmanosteus species rather than the two Australasian Eastmanosteus species and Golshanichthys.
Abstract
Trawl samples along the British Columbia coast between 1999 and 2006 revealed many previously undetected species living in deep water. This increase in knowledge underscores the importance of survey collections for non-game fishes, which form a vital link in marine ecosystems. Although there are few records of albuliform fishes in the eastern North Pacific Ocean, Aldrovandia oleosa (Halosauridae) and Polyacanthonotus challengeri (Notacanthidae) are known from British Columbia. The notacanthid Notacanthus chemnitzii is known from off California, Oregon, and Alaska, but until now it was not confirmed from British Columbia. The ranges of these 3 albuliform fishes are updated in this paper. Until now, 7 species of true eels (Anguilliformes) were known to exist in British Columbia based on literature records and museum specimens; Nemichthys scolopaceus, Avocettina infans, Serrivomer jesperseni, Xenomystax atrarius, Thalassenchelys coheni, Venefica ocella and V. tentaculata. Two synaphobranchids, Synaphobranchus affinis and Histiobranchius bathybius, also occur in adjacent waters of Alaska, and until recently S. affinis was thought to exist in British Columbia based on a misidentified specimen. This paper provides a re-identification of the Synaphobranchus from British Columbia as the 1st record of S. brevidorsalis for the province and also adds Nemichthys larseni and Cyema atrum (Saccopharyngiformes) to the diversity of eels now known from British Columbia waters. We also provide significant range extensions for Serrivomer jesperseni, Thalassenchelys coheni, and Venefica tentaculata along the British Columbia coast.
Abstract
Cusk-eels and brotulas of British Columbia have been poorly studied, and until now, there were published records of only Spectrunculus grandis and Brosmophycis marginata from our waters. However, a single specimen of S. crassus has been identified from among the few S. grandis from British Columbia held at the Royal British Columbia Museum. Furthermore, increased sampling effort from deep-water surveys, shrimp surveys, and the commercial fishery revealed 5 additional cusk-eel species and 1 brotula offshore of British Columbia. Two specimens of Chilara taylori were collected from the southern Strait of Georgia at depths of 78 to 109 m. A single specimen of Acanthonus armatus was taken from near Triangle Island at 1778 m and is the 1st record for the eastern North Pacific Ocean. One specimen of Cherublemma emmelas was found at 1097 m in Kyuquot Canyon, west of Vancouver Island; 4 specimens of Bassozetus zenkevitchi were collected from depths of 1909 to 2125 m west of Vancouver and Graham islands; and a specimen of Cataetyx rubrirostris from 2000 m and a Porogadus promelas from 1967 m were taken in Queen Charlotte Sound, east of the Tuzo Wilson Seamounts. Because of increased sampling effort from 1999 to 2007, we now understand the number of cusk-eels and brotulas in British Columbia to be 9 species.
Abstract
Between 1999 and 2006, the Department of Fisheries and Oceans performed deep-water sampling and discovered new range records for many species of fishes. Here we report 3 species new to British Columbia: Idiacanthus antrostomus, Benthalbella linguidens and Scopelengys tristis, and update the known ranges of 7 additional species (Argyropelecus sladeni, Sternoptyx pseudobscura, Aristostomias scintillans, Opostomias mitsuii, Bathophilus flemingi, Scopelosaurus adleri, and Magnisudis atlantica) in British Columbia waters.
Abstract
Deep-sea anglerfishes were taken in trawls between 1999 and 2006. The 3 most commonly encountered species, Oneirodes thompsoni, O. bulbosus and Chaenophryne melanorhabdus, were known already from British Columbia waters, and here we report significant range extensions for these 3 species. Ceratias holboelli also had been reported from British Columbia, but until now, no specific collection localities had been published. In addition, 3 oneirodids (Oneirodes eschrichtii, O. acanthias and Chaenophryne longiceps), Melanocetus johnsonii (Melanocetidae), and Cryptopsaras couesii (Ceratiidae) are reported for the 1st time from British Columbia.
Abstract
Single specimens of Finescale Triggerfish (Balistes polylepis) and Louvar (Luvarus imperialis) were found in British Columbia’s coastal waters in 2014. Both B. polylepis and L. imperialis normally are found off southern-most California and Baja California. Although a stray B. polylepis was caught as far north as Metlakatla, Alaska, during the 1982–1983 El Niño event, and L. imperialis is known to stray north along the Washington coastline, these 2 new specimens represent 1st records for British Columbia. Both probably moved north during the warm-water anomaly that has persisted along the North American coast since 2013.
Hooker’s Onion post
Allium acuminatum
We are fortunate to have six species of attractive native flowering onions in British Columbia. Nodding onion (Allium cernuum) is widespread. But Hooker’s onion (Allium acuminatum) or taper-tip onion is uncommon BC but widespread on the continent.
Hooker’s onion of the Lily Family (Liliaceae or more recently Amaryllidaceae) grows as a bulbous perennial. The generally creamy to light brown true bulb has the shape of a slightly flattened globe. It is small, less than the size of a thumb nail on average 1.5 cm (0.6″) across. Wild, bulbs occur in clusters of about the size that would fit easily into the palm of a hand. Each bulb bears two to four channeled leaves which are predominantly grey-green with a reddish base. At first the leaves stand erect, but by the time they reach 15 cm (6″) long they reflex. By onion standards, the leaves seem nearly insignificant reaching a maximum of scarcely half a centimetre across and 30 cm (12″) long or less. Leaves usually dry out and break off by flowering time.

Hooker’s onion flower from bulbs grown in pot by Richard Hebda. Photo Dr. Richard Hebda.
Flowers are borne on a firm rounded stalk which ranges from 10-30 cm (4-12″) tall. Two papery bracts surround the bud which contains five to 30 flowers. The blooms sit upon more or less equally long stalklets (called pedicels by botanists), so that the head forms a loose umbel reaching about 7.5 cm (3″) across. Each flower consists of six perianth segments, three petal-like sepals and three petals. The lance-shaped petal-like sepals reach about 1 cm (0.4″) long. Their tips notably reflex especially with age. Six short anthers surround a slightly crested ovary which bears a clearly visible stigma. Mostly the sepals and petals are pink, but may vary from intense rosy purple to nearly white.
In mild coastal climates the first signs of life appear in early February as leaf tips emerge. In Victoria, this occurs well before the end of winter, and sometimes the snow and frost may freeze back young shoots. During April, leaves continue to get longer and reach their maximum length. By the end of the month the first flower stalks poke out of the ground reaching up to 30 cm (12″) tall in June when flowers open. Capsules split in July to reveal black seeds which are easy to harvest by sharply shaking seed heads into a bag.

By Matt Lavin from Bozeman, Montana, USA (Allium acuminatum). CC BY-SA 2.0, via Wikimedia Commons
Hooker’s onion ranges from southern British Columbia to northern California and eastward to Colorado and Wyoming then southward to Arizona. In BC its distribution includes dry parts of Vancouver Island and the adjacent mainland extending into the Fraser Canyon. In our region Hooker’s onion clearly favours dry rocky sites, typically growing in pockets of soil on rocky knolls and coastal headlands. Sometimes it survives in only about 5 cm (2″) of mossy crust cover over bedrock, yet it flowers reliably every year. Occasionally this onion thrives under Garry oaks (Quercus garryana), albeit in very shallow stony soil.
These bulbs are little cultivated and rarely available, yet they thrive under appropriate conditions and produce a pleasing display. The site must be in full sun, sharply drained and with a sandy soil. Avoid summer watering. Rock gardens, the front of dry perennial beds and pots of gritty soil suit Hooker’s onion well in the milder parts of southern B.C.
Plant bulbs about 5 cm (2″) deep about 5 – 7.5 cm (2-5″) apart so that the flower heads touch. Divide the clusters every five to ten years in late summer.
Order Hooker’s onions from specialist native plant suppliers or grow them from fall-sown seed. Do not dig this relatively rare plant in the wild.
First Nations of coastal British Columbia savoured various wild onion species including Hooker’s onion. Bulbs were eaten raw or steamed in great pits. In some areas the pits were lined with pine boughs and covered with lichens and alder boughs. Bulbs and shoots have a mild onion flavour and smell.
Hooker’s onion may be hardy to as low as zone 4 in BC, but its natural distribution suggests zone 5 or higher. We have several native onion species in the Native Plant Garden of the Royal B.C. Museum which flower mainly in June
Cow-parsnip post
Heracleum maximum

Flowerhead of cow-parsnip showing flat-topped form and typical huge leaf bases. Photo Dr. Richard Hebda.
Many of the vegetables we eat came originally from Europe, Asia and Latin America. The aboriginal peoples of British Columbia were unfamiliar with these food plants, nevertheless they feasted on several indigenous green vegetables. The most widely eaten among these was the cow-parsnip (now called Heracleum maximum, recently known as Heracelum lanatum), also referred to as Indian rhubarb or wild rhubarb.
Cow-parsnip belongs to the Parsley Family (Umbelliferae or Apiaceae) and grows in the form of a gigantic perennial herb. A thick hollow stem stands 1-3 meters (40-120”) tall and bears large broad leaves. Stems are lightly ridged and woolly. Each leaf is divided into three segments with coarse teeth. Leaves occur at the base of the stalk and along it. Sometimes you will see big swollen structures at the leaf bases. These are flower buds just waiting to emerge.
The stem top is crowned by several handsome, flat-topped flower heads. Each head consists of numerous umbrella-like clusters of small white blossoms that vary in diameter from 0.5 to 1 cm (0.2-0.4”) across. There are five creamy-white petals in each flower. The blooms circling the outside of each cluster are usually larger and often slightly irregular in form. Five spindly stamens bearing greenish anthers surround a greenish pistil. Cow-parsnip produces robust flattened seeds which remain on the stalk well into the summer.
Cow-parsnip thrives in rich moist soil along streams and rivers, roadsides and in meadows. You will often find it forming large colonies. It has a wide climatic tolerance, growing from sea level to the alpine zone. The Parsnip River in east central British Columbia is named after this plant. Cow-parsnip may be seen almost anywhere in North America in suitable habitats.

Damp alpine meadow habitat of cow-parsnip on the west side of Hudson Bay Mountain near Smithers, BC. Flower-topped stalks of cow-parsnip are visible in the middle of the image to the right of and above patches of purple lupine blooms. Photo Dr. Richard Hebda.
Almost every First Nations group in British Columbia ate cow-parsnip as a green vegetable. Before the flowers appeared in spring, young stalks and leaf stems (petioles) were peeled and eaten raw. Sometimes they were boiled, steamed or roasted. Bruised cow-parsnip plants emit a strong smell but the stems are sweet and juicy, somewhat like celery. Coastal people ate cow-parsnip with eulachon fish grease.
According to Saanich Elders Violet Williams and Elsie Claxton, the stalks had to be collected for eating before the flower buds opened. Otherwise they were tough to chew and tasted too strong.
Be aware that members of the parsley family, especially water hemlock (Cicuta douglasii) and poison hemlock (Conium maculatum) contain terribly strong poisons which can kill a human. You must be certain that the plant you intend to eat is a cow-parsnip.

Peeled young cow-parsnip shoots ready for eating. Photo Dr. Nancy Turner and Robert D. Turner. Used with permission.
Cow-parsnip plants can make a bold addition to your garden but you must give them room. They are best raised from seed, collected as soon as it is mature, and planted in a rich moist soil. You may be able to carefully transplant very young seedlings too, but I suspect that this technique is not often successful.
Cow-parsnip has one characteristic you should be wary of. Like its gigantic relative, giant cow-parsnip or hogweed (Heracleum mantegazzianum), cow-parsnip contains chemicals that may cause severe skin inflammation known as dermatitis. The sap of the plant has particularly strong activity. Ultraviolet rays from the sun activate the compound and may cause the skin to redden and even produce permanent discoloration. Not all people are so affected but be warned to handle the plant carefully especially on a hot sunny day.
The plant supposedly obtained its name after the Greek god Heracles (Hercules). The previously-used species name “lanatum” refers to the “woolly” leaves and parts of the stem.
As you see great stretches of cow- parsnip along our province’s highways, think of it not as a roadside weed but as a valuable food of British Columbia’s First Nations. Cow-parsnip is hardy to zones 2-3 in Canada.

By Dcrjsr – Own work, CC BY-SA 3.0, Link
Mountain Sorrel post
Oxyria dignya
Wild nibbles make a pleasant treat while hiking in the bush. Most often the tasty treat consists of berries of one sort or another, but the occasional green provides a refreshing chew. Mountain sorrel (Oxyria digyna) can spare a leaf or two for the adventurous alpine wanderer.
This delightful hardy herb grows from the top of a tenacious stout tap root. Fleshy, kidney-shaped leaves arise on leaf stalks attached to a short erect stem. Leaf blades range from 1-5 cm (0.4-2”) wide, their stalks 4-8 cm (1.6-3.2”) long. Normally they are coloured bright green but may turn greenish red as the season advances or in really tough sites. There is usually also a single leaf on the stem. The leaves have a sour, but refreshing, acid taste, hence the botanical name Oxyria derived from Greek the word “oxys” which means sharp.

A thriving Mountain sorrel plant showing typical leaves and reddish flowers and fruits. Photo Dr. Richard Hebda.
Like all members of the Buckwheat Family (Polygonaceae), mountain sorrel has small hard- to-see flowers. They cluster irregularly along a 10 to 60 cm (4-24”) tall, narrow flower stalk. Each green to reddish flower consists of four tiny “petals” joined at the base. Two of the petals are keeled, the other two are not. Inside the flowers reside six stamens and a two-parted pistil. Flowers appear from June to August according to elevation and latitude. At maturity, the fruit is broadly winged, turning a showy reddish purple. The fruit is mostly translucent and literally shines when the sun’s light passes through it.
Mountain sorrel ranges throughout the mountains of British Columbia and Alberta, south to New Mexico and California, and north through Alaska and the Yukon and across the Arctic. It also inhabits most of the mountains of Asia and Europe. In our province, mountain sorrel thrives in alpine scree and rock crevices and can be found in suitable habitats on almost every high mountain, to the elevation where no other plants can survive.
Surprisingly this delightful little mountain nibble will grow in lowland rock gardens. It needs a relatively moist gritty run for its root and full sun. In our coastal lowlands mountain sorrel probably needs to be sheltered from full scorching mid-day heat. Plants are best raised from seed sown carefully in the site where it is to grow. Sow the seeds in very stony and moist, but not rich, soil.

The typical harsh high mountain home of mountain sorrel at Shelagyote Peak north of Smithers BC with pink River beauty (Epilobium latifolium) dotting the slope. Photo Dr. Richard Hebda.
Okanagan First Peoples ate fresh raw leaves, but never too many at a time because the oxalic (sour) acid in the plant can be harmful if taken in large quantity. This sorrel contains abundant Vitamins A and C and was used against scurvy in Europe. Like other wild and cultivated sorrels it was widely cooked as a pot herb. A few leaves add a spritely bite when mixed into a salad.
This amazing plant has an incredible story to tell about the glacial history of our province. Studies of the chloroplast DNA by Royal BC Museum and University of Victoria reveal that the genetic makeup of the alpine herb in BC is surprisingly diverse. Within BC, the high diversity and the occurrence of ancient genetic forms suggest that high elevation mountains in the north escaped the last glaciation, contrary to widely accepted thinking.
Mountain sorrel is hardy to zone 0 in Canada. In fact,it is pretty much the hardiest of all plants in the world.
Click here to learn about using DNA to trace the migration of BC’s alpine species*
*Originally published in the Winter 2014 issue of What’s inSight Magazine.
Thimbleberry post
Rubus parviflorus

Highly ornamental flowers of British Columbia’s native thimbleberry. Photo by Dr. Richard Hebda. Saanich, Vancouver Island, British Columbia.
British Columbia is home to shrubs with many uses. For example our Oregon-grapes (Berberis or Mahonia species) make excellent year-round ornamentals, whose fruits produce tasty jelly. Few of our shrubs however can match the thimbleberry of the Rose Family (Rosaceae) for utility. Not only does it have tasty fruits, but this shrub produces edible shoots, soap from its stems, and is an attractive and widely-adapted subject for gardens.
Thimbleberry forms waist- to head-high thickets of numerous erect stems. The stems are thorn-free, unlike the closely related raspberries and blackberries. The bark is distinctively flaky and especially hairy on new growth. Maple-like leaves, 10-30 cm (4-12″) across occur at the ends of the stems. Each one has seven to nine lobes and a texture like soft sandpaper.
Open flower clusters, containing three to eleven blooms develop at the ends of the branches. Each bright white flower can be as wide as 7-8 cm (3″) across. Five long greenish sepals surround five clear white oval petals. A ring of many stamens encircles a central fleshy dome. This dome is the swollen end of the stem, and attached to its surface are numerous tiny greenish pistils. Once the egg in each pistil is fertilized, the pistil transforms into a tiny red fruit with a hard seed inside. The mass of little fruits forms a shallow “thimble” over the central dome, looking like a thin raspberry. The velvety thimbles are somewhat dry but usually taste very sweet.

Thimbleberry shrub blooms. Photo by Dr. Richard Hebda. Saanich, Vancouver Island, British Columbia.
Thimbleberry grows throughout much of British Columbia except the far north. On the continent you can encounter it from Alaska to northern Mexico and eastward to Ontario and Colorado. Typical haunts include open sites, often at the edge of woods, roadsides and shorelines. Surprisingly thimbleberry inhabits both moist and dry sites and occurs across a wide range of elevations from sea level to the high subalpine zone; a widely-adapted plant. Notably, it is one of the species to colonize early after disturbance particularly along highways.
Thimbleberries are excellent subjects for naturalizing in corners of suburban lots. They seem not to be choosy about soil conditions and will grow on raw unprepared surfaces. In fact, in some places they will appear on their own, presumably inoculated from bird droppings. They will grow in full sun to part shade. These shrubs quickly form thickets, generating wildlife cover and stabilize the soil. Butterflies love the flowers and birds relish the fruit.

Delicious fruit of thimbleberry. Photo by Robert D. Turner. Used with permission.
You can often purchase thimbleberries from the local nursery or garden centre by asking them to order it in. There are several suppliers in British Columbia. This native species has recently become available through mail-order from seed and nursery catalogues. Thimbleberries can be raised from seed sown in place in the garden in the fall for germination in the spring. Rooted offset stems will also transplant in a dormant state.
First Nations of British Columbia used thimbleberry for many purposes. Fruits were eaten fresh by most groups or pressed and dried into cakes for later. People of the west coast of Vancouver Island gathered canoe-loads of sweet and juicy spring shoots, peeled and ate them raw. Okanagan people lined their steam-cooking pits with the large leaves. Shuswap Carrier First Nations used the leaves to separate different types of berries in a picking basket. The Cowlitz of nearby Washington State boiled the bark for soap. Today hikers nibble on the wild fruit during their wanderings.
The technical name “Rubus” is based on an ancient Roman name for a related plant. The species name “parviflorus” means “small-flowered”, hardly appropriate for the large attractive blooms produced by thimbleberry.
Thimbleberry is a widely adapted native shrub for most gardens in the province; fruit, vegetable, ornamental and soil healer all rolled into one. It is hardy to Zone 3 in Canada.
Pacific Crab Apple post
Malus fusca

Photo by Gordon Leppig & Andrea J. Pickart. Public Domain.
Have you sometimes wondered what the wild ancestors of our highly-bred food plants may have looked like? The wild apples that spring up in hedgerows on Vancouver Island are often as large as our cultivated forms. Our cultivated crab apples, though they may seem closer to the wild than regular apples, are still the result of breeding. British Columbia’s native Pacific crab apple (Malus fusca or Pyrus fusca), however, may look very much as the ancestors of cultivated apples did many thousands of years ago. Bearing scented blooms, edible fruit and growing to a small stature, it has much potential as a garden and landscape plant.
Pacific crab apple, also known as Oregon crab apple, forms shrubs to small trees from 2 to 12 m (6½ to 40 ft.) tall. Plants branch widely and often form extensive thickets. Distinctive, spine-like, short shoots line the branches but are not nearly as vicious as those of black hawthorn (Crataegus douglasii) or English or common hawthorn (Crataegus monogyna). The grey bark becomes scaly or deeply fissured with age. Oval and pointed leaves look as if they are a cross between those of the domesticated pear and apple. These weakly-toothed leaves sometimes may be lobed near the base.
Delightful blooms have an apple-blossom scent and appear in flat-topped clusters in the spring. Most flowers are white or creamy, but sometimes they take on a warm pink blush and can be very showy. Each flower is about 2 cm (slightly less than an inch) across. The five petals extend well beyond the cluster of stamens in the centre. As in apples and pears, the ovary is inferior, meaning that it is located below, not on the inside of the petals and sepals. In late summer, bunches of oval to cylinder-shaped fruits dangle from long red stalks. Each fruit is about the size of the end of your little finger. An open-grown tree can be “dripping” in fruit similar to some cultivated crab apples. At first the fruits are green and shiny but within a few weeks they turn yellow, pinkish and sometimes even purplish-red. Ripe fruit clusters, especially those well exposed to sunlight, are very attractive. The fruit tastes pleasantly tart when coloured up. After frost it turns brown and mushy but sweet.

Ripening fruits of Pacific crab apple at Island View Beach, Saanich Peninsula, British Columbia. Photo by Dr. Richard J. Hebda.
Pacific crab apple occurs along the British Columbia coast well up many of the main river systems from Alaska to Vancouver Island and on the adjacent mainland to elevations as high as 800 m (2600 ft.). The full geographic range extends all the way from the Aleutian Islands to northern California. The natural habitat tends to the moist side and includes damp woods, stream sides and coastal bogs. These amazing bog trees resemble large gnarled and twisted “creatures” that seem to hail from some distant prehistoric times. They also occur frequently just inland of the ocean shoreline, especially behind beaches and at the edges of estuaries, which suggests that they tolerate salt spray. On some outer coast islets, exposed to the full influence of the sea, our native crab apples may be the only broad-leaved tree among a mass of Sitka spruce (Picea sitchensis) and other conifers.
The hard wood of the Pacific crab apple was widely used along the coast. From it, First Nations people fashioned tool handles, bows, sledgehammers and smaller items, such as spoons and fish hooks. The Nisga’a of northwest B.C. pegged their house boards in place with crab apple wood. The fruit was harvested in early fall and eaten fresh or stored in boxes under water. Apparently, this stored fruit sweetened and softened over time. Medicines, often in combination with other plants, were made from the bark. These medicines were used for a range of internal and external ailments such as stomach problems and skin complaints. The bark and other parts of the tree release hydrogen cyanide, so use only with caution. The flesh of the fruits apparently does not produce much cyanide.
In the garden, Pacific crab apple features best as a specimen tree in an open area. Slow growing, the crown eventually spreads farther than the tree reaches in height. Leaves turn gold and then even red in the fall and combine very attractively with the colours of the ripening fruit. The fruit makes excellent jelly and can be added to other jellies as a natural source of pectin. Wild birds enjoy the ripe fruit, too. Closely planted trees can from a fine dense hedge, and might be good candidates for hedges near the seashore. Plants are best raised from seed sown in the fall in pots and left outside. Seedlings normally take two years to become large enough to plant out.
Up to now the native plant literature has not given much attention to Pacific crab apple, but it has considerable possibilities. The attractive form, flowers and potential for heavy wild fruit production all point to a valuable native species for the garden landscape. Pacific crab apple is hardy to zones 5-6 in Canada.
Yarrow post
Achillea millefolium

Details of yarrow flowers, taken along a roadside in Colwood BC in December. The petals of the white ray florets have been twisted by frost. The brownish grey disk florets are visible in the centre of each little flower head. Photo by Dr. Richard J. Hebda.
Numerous plant species release strong scents when brushed. In the past, these smells were taken as a sign that the plant may have medicinal properties. Even today, some popular remedies still depend on aromatic compounds from plants. For example several brands of cough candies contain derivatives of the eucalyptus plant notable for its distinctly flavoured oil. Many strongly-scented plants thrive in British Columbia, including common or white yarrow (Achillea millefolium), a species well known around the world for its healing value.
Yarrow, a member of the Aster Family (Asteraceae), grows as a herbaceous perennial with leaves and flowers arising from creeping underground or near-surface root stocks. The aromatic fern- or feather-like leaves are finely divided and feel soft. Leaves cluster mostly at the base of the flowering stalk; these leaves are mainly 10-15 cm (4 – 6 inches) long. Many soft small hairs cover the plant making it appear greyish-green.
Tiny flowers crowd into small flower heads, that are further arranged into flat-topped clusters on stalks up to 100 cm (40 inches) tall. Each small flower head in the cluster consists of three to eight tiny ray flowers with a strap-shaped petal, and disk flowers with only reproductive parts. The flowers are white to pinkish and appear from May to October depending on the local climate. On south Vancouver Island they even bloom as late as December. The flower tops dry and turn brown by the end of summer, producing many one-seeded, smooth and flattened fruits.
Yarrow occurs from low to high elevations and often becomes weedy in disturbed settings. This herb thrives in coastal meadows and the arid sage brush steppe of the interior of BC. We also encounter it often in the lower part of the alpine zone during our studies of BC’s mountain flora. You can find yarrow throughout British Columbia and over much of North America. It also occurs across northern and central Europe and Asia.

Yarrow flower head. Photo from page 115 of Saanich Ethnobotany: Culturally Important Plants of the WSANEC People by Nancy J. Turner and Richard J. Hebda.
Yarrow has been valued as a garden plant for centuries. Various forms are available for you to purchase as plants from garden centres and through mail order. Seeds of many colourful selections, especially red and pink shades, are sold through seed catalogues. Plants are easily raised from seed sown into light seed compost or peat pellets in the fall or spring. Rhizome divisions transplant easily too.
Dwarf varieties thrive in rock gardens. Larger types are suitable for mixed borders, perennial beds and the cutting garden. When mowed regularly, yarrow forms a soft-scented ground cover especially valuable for dry lawns. Yarrow is also an excellent subject for the xeriscape garden. This tough plant has been suggested for erosion control on slopes too.

Finely-divided soft leaves of yarrow photographed in January in Saanich BC. Photo by Dr. Richard J. Hebda.
Many First Nations Elders of the B.C. interior value yarrow as a medicine, especially to treat sores. Ulkatcho people of the west Chilcotin soak the leaves in hot water, then apply them in a poultice to sore muscles. This same poultice can also be used to treat saddle sores on horses. Washed and crushed roots were recommended for toothaches. Various teas and concoctions were prepared for internal problems and as a general tonic. Fresh leaves crushed and rubbed on the skin or put to smoke away in a fire act to repel mosquitoes.
Herbal users should be aware that yarrow is “phototoxic.” Skin exposed to crushed or rubbed yarrow may become irritated when exposed to strong sun.
The name Achillea derives from the Greek hero, Achilles, who well knew the medicinal properties of the plant. The finely divided leaves are responsible for the species name “millefolium” meaning “thousands of leaves”. Yarrow is hardy to zone 2 in Canada.
Eriophyllum lanatum
Traditional garden plants often have substitute native species, often hardier, less invasive and easier to manage. The native tall Oregon-grape (Mahonia aquifolium) is an excellent alternative to many cultivated shrubs for mass bedding for example. With a few exceptions, native perennials have yet to replace imports or join the display in more formal settings. Woolly sunflower (Eriophyllum lanatum) is a multi-use native prospect for British Columbia and the west coast of North America.
Woolly sunflower is a spreading fibrous-rooted perennial herb, resembling a restrained version of dusty miller (Artemisia stelleriana). Several hairy stems scramble upwards from the base and bear numerous much-divided silvery leaves. Robust mature plants reach to 60 cm (24”) tall but most often wild specimens rise about 30 cm (12”).

Eriophyllum lanatum (Common woolly sunflower). Photo by Dr. Richard J. Hebda.
Buttery yellow blooms, 5 cm (2”) wide, face brightly upwards like miniature sunflowers. As is typical of the Aster Family (Asteraceae) the flower is actually a flower head of numerous florets. The rays or outside flowers have narrowly oval petals 1-2 cm (0.4 -0.8”) long. About 8-13 of these frame a disc of tightly-packed inner florets without the showy rays, just as you find in a typical garden marigold. Flowers are borne singly on long stems rising well above the silvery blue foliage. Flowers appear from early May to August, generally in June in southwest BC.
Woolly sunflower populates relatively dry open habitats such as bluffs and rocky slopes, largely being confined to low to mid elevations. In BC, it ranges along the coast southward from Vancouver Island and the adjacent mainland, and inland to the Fraser Canyon with a population in the southern Interior. In the United States, the range extends well into California and eastward to Montana and Wyoming. The inland occurrences of woolly sunflower suggest a strong potential for BC interior gardens.
This marvellous plant has several garden uses. First, it thrives in the dry and sunny rock garden, even on poor stony soil. Although it may take a year or two, your plants will become established, persist, and flower from year to year. A patch placed in raw subsoil mixed with gravel has grown for more than 15 years at the Royal BC Museum Native Plant Garden. I have spread it widely along my driveway where it thrives! Woolly sunflower grows well in pots too, as a perennial surrounded by annuals planted freshly year after year. April Pettinger and Brenda Costanzo in their excellent book Native Plants in the Coastal Garden recommend woolly sunflower for shoreline plantings, use in repeated drifts, and in containers. They also note its resistance to deer.

Eriophyllum lanatum (Common woolly sunflower). Photo by Dr. Richard J. Hebda.
Woolly sunflower is grown in many native plant nurseries especially in the western United States and is known even in Europe. You can propagate it by root cuttings in late winter, seeds sown in fall and crown divisions. I multiply my woolly sunflowers by pulling out rooted stems or digging out small plugs at almost any time of the year and replanting. Once in while pull out invading grasses and other weeds from the spreading mats.
The First Nations people of the Thompson River region knew this species as either yellow flower or as “friend or relative” of the much larger and related Balsamroot (Balsamorhiza sagittata). In Washington State, dried flowers were used as love charms and the leaves could be rubbed on one’s face to prevent chapping.
Eriophylllum lanatum is also known as Woolly Eriophyllum and Oregon Sunshine. In Canada it is hardy to Zone 5 meaning it likely will grow in many gardens throughout the south.
Take a chance on the wild side and try growing woolly sunflower in your garden. Experiment with it as a replacement for silvery-leaved ground covers and enjoy the annual display of golden blooms.
Crowberry post
Empetrum nigrum
Heathers (Calluna species) and heaths (Erica species) are popular ground cover plants worldwide. True heathers and heaths are not native to British Columbia, but many heather relatives and heather-like plants thrive in our province, some under harsh inland climates. Crowberry (Empetrum nigrum) (also known as curlew-berry and crake berry) is an excellent, extremely hardy, heather substitute that fulfils the role of ground cover and produces abundant attractive black berries.
Crowberry plants consist of scrambling woody stems rising to 20 cm (8″) tall, and enveloped in wooly hairs and short evergreen leaves. Small roots anchor the stems into the soil. Often a mass of better developed roots associated with a loose crown in the middle of a patch provide a firm grip. Numerous needle-like leaves are arranged in alternate fashion or grouped in fours. These grooved leaves extend 4-8 mm (0.15-0.3”) long and have their margins rolled under.
Tiny purplish flowers appear early in spring, scattered along the stem at the base of the leaves. There are male and female flowers, which sometimes occur on separate plants. Each flower has either one tiny ovary, or three purple stamens, sometimes with up to three purplish petals, all cupped in tiny sepals and bracts.
Unlike the inconspicuous blooms, the shiny black bead-like fruits are easily visible and very attractive. These globe-shaped berries are fleshy and juicy, about half a centimeter (1/5 “) across. On loose leafy plants the berries often occur singly, spaced along the stems. On low growing plants they cluster in bunches and appear to nearly smother the plant. The berries contain large white seeds and are a favourite food of bears.

Ripe crowberry fruits growing on a dense mat of stems and leaves in the high mountains of northern British Columbia. Photo by Richard Hebda.
Crowberries are widespread plants with a most hardy constitution. You can find them almost anywhere in our province in suitable habitats except in the dry lowland climates of the southern interior. Plants have been observed to nearly 2500m (about 7750 feet) above sea-level in BC. Crowberry’s geographical range extends across all of Canada, southward on the west coast to California and around the northern hemisphere. It is largely a plant of full sun environments. At low elevations, especially on the coast, it inhabits bogs and openings in bog forests. Inland you may encounter it in conifer forest openings and almost everywhere in the alpine tundra and subalpine turf as well as on dry rocky mountain knolls. Crowberry favours acid to very acid soils usually with abundant organic debris.
This creeping shrub has great potential as a ground cover species in most BC gardens. It is especially suited to interior gardens in regions with cold harsh climates. On the coast it is better suited to open moist rock gardens or bog gardens where it will grow into a loose luxuriant mass of heather-like stems. Plants are most easily grown from root-bearing stem pieces planted in moist sandy peat soil until well rooted. Nursery material has also been grown from cuttings and fresh seeds sown in the fall. Crowberry is hardy to zone 1 in Canada.

A carpet of pure crowberry stems and leaves richly covered in berries, northern BC. Photo Richard Hebda.
Crowberries were eaten widely throughout British Columbia. Anyone who has munched a crowberry on a wilderness hike knows they tend to be somewhat watery and slightly tart, but pleasant nevertheless. On the coast crowberries were mainly eaten fresh by Haida and Tsimshian peoples in whose lands they can occur in great abundance. Haida Elders thought that eating too many of them might lead to internal bleeding. In the northern interior many First Nations people ate the berries because they are available all winter long under the snow. Some Carrier people mixed the berries with bear grease. Berries were also prepared by mashing them and cooking with heated stones in spruce bark troughs. Dried mash was soaked in water and consumed later. Mixed with sugar the berries can be used for pies and jellies.
Crowberries are an excellent heather-like ground cover for coastal and especially cool interior gardens, despite the absence of showy flowers. And unlike true heathers you can nibble tart fruit in fall and even in winter. So, enjoy this widespread native in the wild and maybe find a spot for it in your garden.
Alaskan Bunchberry post
Cornus unalaschkensis
British Columbia forests are renowned for the trees they grow. Within these great forests there are other botanical treasures that live on the forest floor. Among these the bunchberries (Cornus spp.) are among the most widespread.
Bunchberries belong to the Dogwood Family (Cornaceae) along with our provincial floral emblem the western flowering or Pacific dogwood (Cornus nuttallii) and red-osier dogwood (Cornus stolonifera). Bunchberries grow as low, carpeting herb-like shrubs. A root-stem system (rhizome) spreads just below the ground surface and from it arise 5-20 cm (2-8″) tall flexible stems. Each erect stem bears a pair of small highly reduced leaves about halfway up, and a whorl of 2-8 cm (0.8-3.2”) long oval leaves at the top. The leaves are usually dark green and somewhat glossy but may yellow in full sun. The veins appear to be well pressed-into the leaf surface. Tear through the leaf and you will see several thin whitish strands along the torn edges, a characteristic of dogwood leaves.
A flower head develops in late spring to early summer at the top of the stem. Four to six modified leaves called bracts surround the cluster of tiny flowers in the centre. Most people think that these white bracts are the petals but they are not. True flowers huddle cheek-to-jowl in a central clump. Each mini-bloom consists of a tiny toothed tube of greenish sepals which in turn surrounds a tiny funnel of four purplish petals. The pistil hides within the throat of the flower. Four spindly stamens poke out from the mouth. The berry-like fruits mature in late summer and early fall into a bright red bunch (hence the common name). Alaskan bunchberry differs from the common and widespread bunchberry (Cornus canadensis) which has greenish white petals and no well-developed leaves on the stem.

By Walter Siegmund (talk) – Own work, CC BY-SA 3.0, Link
Alaskan bunchberry haunts moist old-growth forests and thickets of British Columbia’s coastal strip. It thrives on acid soils rich in humus, draping over rotting logs and crowding under shrubs at the edges of bogs and in the sub-alpine zone. The geographic range of the species extends both north and south along the coast into Alaska and the northwestern United States. Common bunchberry replaces Alaskan bunchberry east of the Coast Mountains.
Alaskan bunchberries were much savoured by First Nations people of the coast. The berries were eaten raw, with eulachon fish grease and with sugar. Haida occasionally steamed the fruit, mixed it with water and grease and stored it for the winter. Although the berries have a pulpy texture and a large seed, their taste is pleasantly sweet.
Bunchberries make excellent garden subjects especially in moist shaded settings under trees and on the north side of buildings. They combine well with shrubby members of the heather family (Ericaceae) such as rhododendrons or azaleas. They need moist, airy and humus-enriched soil to thrive and do not enjoy warm sunny settings. We have grown bunchberries with tall ferns under a tree in the Native Plant Garden of the Royal British Columbia Museum but they never seemed to flower the way they do in the wild.

Bunchberry Fruit. By Aarongunnar – Own work, CC BY-SA 3.0, Link
Raise Alaskan bunchberries from seed or buy them from a garden centre or nursery where they are occasionally available. Sow the seed in the fall in a pot of peaty soil and leave over the winter. Plants with a vigorous root system raised in a pot succeed best.
The name “Cornus”, an ancient name for dogwoods, may be derived from “cornu” an old name for horn, because of the very hard wood of some of the tree dogwood species. The species name “unalaschkensis” recalls that the plant was named after Unalaska Island in the Aleutian Islands.
Saskatoon post
Amelanchier alnifolia

Saskatoon Berries. By Walter Siegmund (talk) – Own work, CC BY-SA 3.0, Link
Northern North America is not known as a source of important of fruit crops. Most of our familiar fruits such as apples, plums, and cherries originated elsewhere. Several of our native species, such as coastal strawberry (Fragaria chiloensis) have been used in breeding programs. However, Saskatoon (also Saskatoon serviceberry), a showy shrub of the Rose Family (Rosaceae), provides wild fruit and is now grown as a commercial crop.
Saskatoon grows as a medium shrub to small many-stemmed tree reaching to 7 m (23’) high. The smooth stems are reddish brown to dark grey, and young twigs often silky. The 2.5 – 3.0 cm (1-1.2″) long leaves have an oval outline, but may be slightly pointed at the tip and heart-shaped at the base. Small teeth line part, or all the leaf’s margin. Young leaves are bright green but turn bluish green with age.
Bright white flowers occur in leafy clusters toward the ends of the branches. Somewhat hairy sepals form a base for the 1-2 cm (0.4-0.8”) long strap-shaped petals. About 20 stamens choke the throat of each flower, where they surround four to five styles.
The ovary of Saskatoon, like that of the apple or pear, is placed in an inferior position. This means that the sepals and the petals arise from the top of the ovary rather than below it, as is the case in a superior ovary.
The fruit is about 0.5 to 1.0 cm (0.2-0.4”) across, and globe shaped. Its colour ranges from purple to nearly black, often covered by a greyish blue bloom. Good quality fruit is juicy and sweet, however on the coast some of the berries dry out quickly becoming mealy or crunchy with little flavour.
Saskatoons thrive throughout British Columbia. The continental range extends along the coast from Alaska to California, eastward to New Mexico and north through the plains and prairies into Canada’s Northwest Territories. This shrub favours open to lightly shaded sites such as thickets, fence rows, clearings and edges of woods. A well-drained soil is essential.

Mid spring flowers of Saskatoon. Photo by Dr. Richard Hebda.
Saskatoons were and are widely picked by First Nations. The Nlaka’pamux (formerly the Thompson) people recognized several types of bushes. Some types were gathered and dried for winter use. Other types were cooked to a jam-like form before being dried. Edible roots of other plants were sometimes soaked in Saskatoon juice to make them more flavourful and sweeter. Dried and rehydrated berries were added to dried vegetables and cooked into soups and puddings.
Many parts of the Saskatoon plant were used. A drink was made from the bark for stomach problems. Bark and twigs were turned into a medicine for recovery after childbirth and, in combination with other plants, to make a contraceptive. The tough hard wood made excellent arrows. Other uses included digging sticks, spear shafts and handles for tools. Saskatoon sticks were used to spread out cleaned salmon for drying, and the branches to construct shelters.
Today many British Columbians eat fresh berries off the bush or bake them in tasty pies. You can buy Saskatoon bushes specially bred for the home garden. These varieties produce bigger and sweeter fruit than most wild plants. In the prairies Saskatoon plantations yield the raw material for a regional specialty, Saskatoon wine.
Growing Saskatoons is an easy matter if you have a sunny well-drained site. In the late winter or early spring, buy a bush from the garden centre or order it through the mail. Plant the sapling in a moderately rich, but not heavily-fertilized, soil and mulch around the base. You may have to water during the first year to help the young plant settle in. Saskatoon shrubs can be used in many parts of the garden; the spring flowers are stunning and the bright yellow to red fall leaves provide a cheerful accent. Plants can be grown from seed and dug up as self-sown seedlings too.
The origin of the name Amelanchier remains unclear. The species name “alnifolia” means alder-leaved.
Saskatoon is an outstanding native shrub widely adapted to B.C.’s varying climates. Not only does it yield tasty fruit, but it serves well as a showy garden subject. To see Saskatoon berries visit the Native Plant Garden of the Royal British Columbia Museum, Victoria, B.C.
June Plum post
Oemleria cerasiformis

Male flowers of June plum. Photo by Dr. Richard Hebda.
Spring arrives early in southwestern British Columbia, and many plants waste no time producing flowers. June or Indian Plum, known botanically as Oemleria cerasiformis or not long ago as Osmaronia cerasiformis, is one of the earliest of all shrubs to bloom.
June Plum (also called Oso berry) is a deciduous shrub in the Rose Family (Rosaceae), which grows from 2-5 m (6.5-16.5’) tall. Stems and old branches are grey, but young branches appear dark wine red. The branches form a graceful upswept pattern. Thin, bright green leaves unfold as early as February. Tapered and oblong, they are usually widest above the mid-point. The leaf margin is smooth-edged and the lower surface paler than the upper. Leaves alternate along the branch, often standing erect when young. Crushed leaves smell like cucumber or watermelon rind.
Clusters of flowers dangle from the branch tips as the first leaves appear. A fully open flower cluster may reach 10 cm (4″) long and have six to 12 blooms. Each greenish-white blossom is about 1-1.5 cm (1/4-1/2″) across. Some people describe the flowers as almond-scented, whereas others call them bad smelling! In any case, once you have smelled them, their scent will be forever recognizable.
Five small greenish sepals and five greenish-white rounded petals arise from the edges of a cup-shaped structure, called a hypanthium. The hypanthium is formed from the swollen end of the stalklet bearing the flower. Stamens or pistils reside in the cup of the flower with male and female flowers on separate plants. In the male flower, 15 stamens form three rows at the edge and just inside the cup. Five pistils crowd together in the bottom of the female flower cup.

Early March display of June plum, Saanich Peninsula, Vancouver Island. Photo by Dr. Richard Hebda.
Male plants, perhaps surprisingly, produce the most abundant and showiest flowers. Having male and female flowers on separate bushes reduces the chances of in-breeding. But a great mystery surrounds the question of pollination. At flowering time there are few if any, bees out. Possibly native beetles do the honour, but even after many hours of observation, the responsible party has yet to been seen for sure.
Small fruits appear in spring. At first these are a pleasant peach colour but soon darken to dark blue or nearly black. Like their distant relatives, the true plums, the fruits are covered in a greyish bloom. A prominent groove marks one side of the fruit. Birds especially Cedar Waxwings gobble up ripe fruit and disperse the seed.

Immature bitter fruits of June plum. Photo by Dr. Richard Hebda.

These fully-ripe June plum fruits are far less astringent than peach coloured ones. Photo by Dr. Richard Hebda.
The distribution of June plum, hugs the southwest coast of British Columbia, on south Vancouver Island north to about Campbell River, and on the mainland as far as Yale and Squamish. Moist thickets, open woods and stream banks are its favourite haunts. June plums occur along the Pacific Coast, west of the Cascade Mountains as far south as northern California.
Where the climate permits, June plum makes a fine early spring shrub for the wild or woodland garden. It thrives in the Native Plant Garden of the Royal British Columbia Museum where it requires little maintenance. The coloured fruits also draw much attention in early summer.
For flower and fruit display you will need one male and one female plant. This shrub is raised from suckers, twig cuttings or seeds. Seeds need a long, cold moist spell followed by a warm interval to germinate, so best plant them in the summer when fruit ripens and leave until the following spring. You may often find seedlings in fence lines and shrubs where birds perch and pass seed.
Generally, the fruit has a bitter choking taste, though I find fully ripe fruit tasty. Nevertheless, First Nations people around the southern Strait of Georgia and Puget Sound ate small quantities of fresh or dried “plums”. The fruit is not poisonous, although at the Royal BC Museum we get calls from concerned parents whose children have tried it. First Nations of the Saanich Peninsula still boil Indian plum bark (sticks) in water and drink the liquid for diarrhea. Bark is also used in a complex mixture to treat several serious ailments.
Oemlaria got its name from an obscure individual called “Oemler”. The species name cerasiformis refers to the cherry-shaped appearance of the fruits.
See June plum in the Native Plant Garden at the Royal BC Museum at any time of the year. It is at its best in the warming days of late February and March when the first flowers open to greet the sun.
Bigleaf Maple post
Acer macrophyllum

Hanging flower clusters of bigleaf maple. Note the protruding stamens. Photo by Dr. Richard Hebda.
We have long recognized the vital role of shade trees in creating a pleasant environment around our homes. Most shade tree species and varieties hail from distant regions and lands. British Columbia is home to one of the noblest, but for some reason little-used shade trees, bigleaf maple.
Bigleaf, broadleaf, common or Oregon maple grows as a tall spreading tree to 30 m (100′) high, casting filtered shade beneath. In undamaged trees, the widely spreading root system supports a short trunk 60-150 cm (2-5′) across, from which reach out great limbs. However many trees have been cut, and hence grow as groups of tall greyish sapling stems. Old branches are bedecked and festooned with colourful mosses, leafy and crusty lichens. Young twigs are coloured an attractive medium green. Fat green buds end the branches during the winter.
Leaves of bigleaf maple are the largest of any tree in British Columbia. Their form is typical for maples; three to five sharply-tipped lobes with deep indentations between them. Spring foliage emerges soft yellow green in the sun’s light and soon expands to full size, some leaves more than 30 cm (12″) across. In fall the foliage mantles each tree in a rich yellow-orange cloak, before the leaves fall to earth to be crunched underfoot.
The 10-15 cm (4-6″) long flower clusters rival any in the genus of maples. They burst forth in early spring revealing often 50 or more small greenish yellow fragrant blooms. Male and female flowers are separate, but occur in the same cluster. Each flower consists of petal-like sepals, and five petals surrounding either a group of long protruding spindly stamens or a two-parted ovary with two stigmas. The clusters magnetically attract pollinating flies until each tree hums softly like a giant machine. The large fruits have the typical form of maple keys consisting of a wing and body. The spiny hairs, which cover the surface of the body, will penetrate and irritate the skin.

Summer leaves of bigleaf maple. Photo by Dr. Ken Marr.
In British Columbia the maple’s natural range includes most of Vancouver Island and the adjacent mainland coast, extending well up major valleys. Bigleaf maple is reported as far north as Alaska, but the main distribution extends from BC mostly west of the Cascade Mountains south to California with an outlying population in Idaho. Bigleaf maple thrives in rich moist soils especially along rivers, streams and floodplains. Curiously you will also find it on moist rocky slopes often rooted in the rubble at the foot of cliffs. The tree grows well in disturbed settings along roads and fence rows.

Late summer paired maple keys, the fruit of bigleaf maple. Photo by Dr. Ken Marr.
Although some authors consider bigleaf maple of little horticultural value, it makes an outstanding shade tree. The airy canopy produces light, rather than oppressive shade. Once the leaves fall, weak but welcome winter sunlight can penetrate into the home. Because of its size, this maple may not suit refined urban gardens, but it cannot be beat as a huge specimen tree for a large yard, park or street planting. You can easily raise maple trees from seeds planted in ordinary soil. Often, hundreds of seedlings struggle to survive under majestic parent trees. These seedlings transplant easily in the moist coastal winter and spring. You may have a problem growing a traditional flower garden below the thirsty tree. However, native species such as salal (Gaultheria shallon), Oregon Grape (Mahonia spp.) and sword fern (Polystichum munitum) thrive beneath its umbrella.
Bigleaf maple wood found many uses among British Columbia’s coastal First Nations. From it they made dishes, spoons, rattles, bark shredders, axe handles and numerous other tools. The wood is perhaps best known for the carving of beautiful spindle whorls and canoe paddles. Many aboriginal peoples valued the wood as a fuel, for it burns hot and clean. Bigleaf maple is an excellent fuel tree today too, because it can be cut to harvest the firewood and re-sprout fresh new suckers for future wood production. Well-managed root systems and stumps yield firewood for many decades. Aboriginal people also used the bark to weave ropes and baskets. The large leaves were spread over and under food in steam pits and picking baskets.
Incidentally people have tried to make syrup from spring sap but the sugar concentrations are mostly too low and the day-night temperature changes too slight for a good flow.
The scientific name Acer derives from the classic Latin name for maple. The species name “macrophyllum” celebrates the tree’s most obvious feature, the big (macro) leaves (phyllum). This handsome arboreal giant and other maples were once happily in the Maple family (Aceraceae). Now through the wonders of DNA analysis they have been added to the Horse-chestnut family Sapindaceae.
Looking for a shade tree or an excellent permanent firewood source, try our native bigleaf maple. This under-appreciated native species deserves to be more widely grown. For more information on native species please visit our Native Plant Garden, of the Royal British Columbia Museum in Victoria.

Photo by Dr. Richard Hebda.
Sedum spathulifolium
British Columbia is full of rocks, and rocks are not hospitable places to make a living. One group of plants, the stonecrops (Sedum spp.), have adapted superbly to this often harsh setting. Broad-leaved (also broadleaf) stonecrop (Sedum spathulifolium) is an exceptionally attractive and abundant stonecrop reveling in the stony outcrops on the coast of our province.
Broad-leaved stonecrop of the Stonecrop Family (Crassulaceae), forms low, spreading mats in patches of densely-packed rosettes of flattened leaves. These leaf rosettes arise along a creeping stem called a stolon. Clusters of fibrous roots from the stem cling tenaciously to rocks and shallow soil spreading from crevice to crevice. About 15 leaves form the rosettes which typically reach 2-4 cm (0.8-1.6″) across. The fleshy, greyish-green leaf blades sit almost directly on the stem. Each leaf broadens out wider than the base to a broad tip, hence the common name. Like many plants which survive in harsh dry places, this herb stores water in its thick leaves.
Brilliant yellow flower heads adorn stiff stalks which stand about 10-15 cm (4-6″) tall. Small five-petalled star-like flowers crowd the flower head. The narrow pointed petals surround a cluster of 10 yellow stamens 5 pistils. In British Columbia, the flowers appear mostly from May to July.
Broad-leaved stonecrop grows abundantly on coastal cliffs and dry sites on Vancouver Island and adjacent Gulf Islands but less frequently on the nearby mainland. The range extends southward as far as California. Look for stonecrop along the shore and even well up mountain slopes in dry forest openings.

Photo by Dr. Richard Hebda.
This stonecrop excels as a garden plant for dry sites. Simply plant a small piece of rooted stem in a little bit of soil. Try it in a rock garden, on a stone wall or next to a stone or gravel path. Even tuck it into a roadside rock face. Can you image what our rocky roadsides might look like covered in brilliant yellow? Once this plant takes hold, it will spread in a most attractive manner plugging cracks between rocks and blanketing rough spots. This species tolerates some shade too, although its form becomes generally looser. Stonecrop needs little attention once established; remove dead stems after flowering if you wish, and keep grasses from rooting in the mat. As the patch expands you will be rewarded with plenty of offsets for new plantings. I have watched a small piece double in size after a month and quadruple after a season. Broad-leaved stonecrop is one of the easier native plants to get because it is occasionally available through garden centres. Indeed, several forms, including some with reddish or purplish leaves, others with a bluish hue, are being sold. A grey-white variety called ‘Cape Blanco’ seems to be widely available. You could create a rock garden of varying hue by planting several different colour forms. Broad-leaved stonecrop is hardy to zones 5-6 in Canada.
First Peoples put stonecrop to some interesting uses. Nlaka’pamux (previously Thompson) People of the Fraser River Canyon boiled the whole plant and used the liquid to soothe cross babies and relieve constipation. Stonecrops, in general, were used to make poultices for treating piles.
The scientific name Sedum apparently was used for stonecrops in ancient times, possibly derived from the Latin “sedeo” which means “I sit” as indeed it does on its favoured stony substrates. Come and see broadleaf stonecrop sitting around on the rocks in the Native Plant Garden of the Royal British Columbia Museum in Victoria.
Tall Oregon-grape post

Mahonia aquifolium shrub. The original uploader was Meggar at English Wikipedia – Transferred from en.wikipedia to Commons., CC BY-SA 3.0.
Mahonia aquifolium
British Columbia is home to a diverse collection of gorgeous native shrubs. Many of them produce edible wild fruit too. Few are as versatile and adaptable to the home garden as the Oregon-grapes (Mahonia spp.) and of these Tall Oregon-grape (Mahonia aquifolium) has the highest profile.
Tall Oregon-grape forms handsome shrubby clumps of few to many upright greyish canes. Wild clumps normally grow half to one and a half metres (20-60″) tall, but straggly individuals can reach 2.5 m (100″). The inside of the stems and roots is coloured brightly yellow. Shiny, compound evergreen leaves, with 5-9 leaflets crowd toward the ends of stems. Spiny teeth line leaflet edges giving rise to the species name “aquifolium” which is a classical name for holly (Ilex spp). Hollyleaved barberry is another name for the plant and as is the case with the holly the leaves can be painful. In the summer, leaves shine lustrous green, but in the winter they turn an attractive bronze or even red. Delicate new spring growth emerges reddish too.
On the coast many-flowered clusters of brilliant yellow blossoms appear as early as late February and persist into April. Often several clusters throng the end of a stem creating a colourful display. In the blooms of this plant, the petals and sepals are both coloured so that each delicately scented flower has three whorls of three petal-like segments. Glands at the base of the segments produce clear drops of nectar. Inside a ring of seven yellowish stamens, each with peculiar projecting “ears”, squats a greenish pistil. Grape-like bunches of small blue berries replace the flowers in late spring and summer. The stunning display of abundant fruits is so appealing that you might be tempted to stuff them into your mouth. Avoid that temptation because the berries are mouth-pucker sour and contain large seeds.

Mahonia aquifolium fruit. Photo by Dr. Richard Hebda.
In British Columbia this shrub occurs from Prince George southward in the interior and to the north end of Vancouver Island on the coast. The range extends southward into Oregon and eastward to Idaho. The natural habitat includes open woodland, edges of meadows and, in the interior, sagebrush covered hills.

Top: Mahonia aquifolium flower. By Walter Siegmund (talk) – Own work, CC BY-SA 3.0, Link. Bottom: Mahonia aquifolium flower. By Aelwyn – Own work, CC BY-SA 3.0, Link
Tall Oregon-grape was introduced to European gardens in the early 1800s. Generally it is a co-operative horticultural shrub especially for partly-shaded to open well-drained sites. Once planted it may need occasional pruning immediately after flowering to keep a neat form. Cold, drying winter winds kill exposed foliage. Try tall Oregon-grape in a shrub border, hedge, or naturalized thickets along with Nootka rose (Rosa nutkana) and snowberries (Symphoricarpos spp.). Landscapers use it widely around institutional buildings on south Vancouver Island. A dense hedge of it may keep out nosy intruders.
Tall Oregon-grape plants are easily obtained from garden centres and nurseries on the west coast. They can be grown from seed too. Sow ripe seed in the fall and transplant young seedlings the following fall into nursery beds. At the Native Plant Garden of the Royal British Columbia Museum tall Oregon-grape spreads widely by seed into adjacent herbaceous beds. Thickets spread outward underground so prepare to keep them in check.
First Peoples knew tall Oregon-grape well. They ate the fresh berries and, on south Vancouver Island, used them as an antidote to shellfish poisoning. Nlaka’pamux (previously Thompson) People of the Fraser River Canyon boiled the outer bark of the roots to make a bright yellow dye for baskets. Liquid from the bark of boiled woody stems helped treat red itchy eyes.
The blue berries make an excellent wild jelly. Use lots of sugar to moderate the tartness, and screen out the large and bitter seeds.
Our Oregon-grapes have been known botanically by two names: Mahonia and Berberis. Mahonia, honours an 18-19th century American horticulturalist Bernard M’Mahon. The name Berberis (barberry in English) originates in a similar Arabic name for barberry fruit.
Tall Oregon-grape is a wonderful coastal landscape shrub. It is attractive all year round and produces edible berries. Look for it and its berries in the wild and come and see masses of it at the Native Plant Garden of the Museum in Victoria.
There isn’t a month of the year where European Wall Lizards are completely torpid here on Vancouver Island. We are nearing the solstice, and lizards are still out and about.
 I went out with my wife and daughter to Crumsby’s this weekend, and later, we had plans to do some shopping – and groceries – the usual weekend activities.
I went out with my wife and daughter to Crumsby’s this weekend, and later, we had plans to do some shopping – and groceries – the usual weekend activities.
But the weather was nice – 7 degrees Celsius and sunny – so I suggested we divert to the Moss Rocks area for a few minutes and see if we could find a European Wall Lizard. My wife now accepts such requests as normal – we may be talking about christmas shopping and trying to entertain our daughter, but she knows I am looking for lizards if it is sunny. At least lizards in a cup, tub, or zip-lock bag don’t smell as bad as some of the road-kill we have collected.
I saw – or more accurately heard – the first lizard just as I stepped out of the car. Within seconds I had evidence of lizards active in December. They were high up on the bedrock wall along May Street. A patient wait of a few more minutes was rewarded by sight of three lizards which crawled out of a cold crevice, and lined up to absorb as much heat as possible from the sun.
 Farther along the street was a concrete wall covered in roots – an easy place to ambush a lizard. There were at least 8 lizards on that small concrete wall – I caught these three in two minutes. They now are preserved a cataloged as 2127 in the Royal BC Museum’s herpetology collection as evidence of the winter activity of an invasive lizard.
Farther along the street was a concrete wall covered in roots – an easy place to ambush a lizard. There were at least 8 lizards on that small concrete wall – I caught these three in two minutes. They now are preserved a cataloged as 2127 in the Royal BC Museum’s herpetology collection as evidence of the winter activity of an invasive lizard.
Interesting tidbit of information was that the lizards were warm to the touch – they must find really effective basking sites to warm themselves that much above the ambient air temperature. The rocks themselves were not that warm.
Michael Cheney, Kendrick L. Marr
We report the recent discovery of Oxypolis occidentalis, a species that is new to both British Columbia and Canada, disjunct on the Queen Charlotte Islands.
Kendrick L. Marr1, Geraldine A. Allen and Richard J. Hebda
Aim Late Pleistocene ice sheets are thought to have covered most of western Canada, including all of British Columbia (BC). We examine patterns of genetic variation in an Arctic–alpine plant to evaluate the possibility of full glacial refugia within the area covered by the Cordilleran ice sheet (CIS) and to uncover post-glacial migration routes.
Location Western North America.
Methods We sampled 1030 individuals of the Arctic–alpine plant Oxyria digyna from 117 populations distributed over much of its range in western and northern North America. DNA haplotypes were identified using restriction site analysis of two chloroplast DNA intergene spacer regions, psbA-trnH and trnT-L. We examined the geographical distribution of haplotype diversity in relation to latitude, and also compared various indices of diversity in putatively glaciated and unglaciated regions. Patterns of migration were inferred using nested clade analysis.
Results We detected a total of 20 haplotypes. High haplotype diversity was found in Beringia, in unglaciated western USA, and in northern BC at 57–59° N, well within the accepted limits of the CIS. Ancestral haplotypes were also centred in northern BC.
Main conclusions High genetic diversity of Oxyria digyna is expected in unglaciated regions, but unexpected in northern BC if British Columbia was entirely covered by ice during the late Pleistocene. Our observations suggest the presence of unglaciated areas providing late Pleistocene refugia in northern BC. Such refugia would have important implications for the origins and migrations of many plant and animal species in north-western North America.
Allen, GA, Marr, KL, McCormick LJ, Hebda RJ
The ranges of arctic-alpine species have shifted extensively with Pleistocene climate changes and glaciations. Using sequence data from the trnH-psbA and trnT-trnL chloroplast DNA spacer regions, we investigated the phylogeography of the widespread, ancient (>3 million years) arctic-alpine plant Oxyria digyna (Polygonaceae). We identified 45 haplotypes and six highly divergent major lineages; estimated ages of these lineages (time to most recent common ancestor, T(MRCA)) ranged from ∼0.5 to 2.5 million years. One lineage is widespread in the arctic, a second is restricted to the southern Rocky Mountains of the western United States, and a third was found only in the Himalayan and Altai regions of Asia. Three other lineages are widespread in western North America, where they overlap extensively. The high genetic diversity and the presence of divergent major cpDNA lineages within Oxyria digyna reflect its age and suggest that it was widespread during much of its history. The distributions of individual lineages indicate repeated spread of Oxyria digyna through North America over multiple glacial cycles. During the Last Glacial Maximum it persisted in multiple refugia in western North America, including Beringia, south of the continental ice, and within the northern limits of the Cordilleran ice sheet. Our data contribute to a growing body of evidence that arctic-alpine species have migrated from different source regions over multiple glacial cycles and that cryptic refugia contributed to persistence through the Last Glacial Maximum.
KEYWORDS:
Arctic-alpine plants; Pleistocene glaciations; cpDNA; phylogeography; refugia
Wang, Q, Liu J, Allen GA, Ma Y, Yue W, Marr KL, Abbott RJ
Many plant species comprising the present-day Arctic flora are thought to have originated in the high mountains of North America and Eurasia, migrated northwards as global temperatures fell during the late Tertiary period, and thereafter attained a circumarctic distribution. However, supporting evidence for this hypothesis that provides a temporal framework for the origin, spread and initial attainment of a circumarctic distribution by an arctic plant is currently lacking. Here we examined the origin and initial formation of a circumarctic distribution of the arctic mountain sorrel (Oxyria digyna) by conducting a phylogeographic analysis of plastid and nuclear gene DNA variation. We provide evidence for an origin of this species in the Qinghai-Tibet Plateau of southwestern China, followed by migration into Russia c. 11 million yr ago (Ma), eastwards into North America by c. 4 Ma, and westwards into Western Europe by c. 1.96 Ma. Thereafter, the species attained a circumarctic distribution by colonizing Greenland from both sides of the Atlantic Ocean. Following the arrival of the species in North America and Europe, population sizes appear to have increased and then stabilized there over the last 1 million yr. However, in Greenland a marked reduction followed by an expansion in population size is indicated to have occurred during the Pleistocene.
KEYWORDS:
ancestral location; arctic flora; circumarctic distribution; migration; species origin
Kendrick L. Marr, Geraldine Allen, Richard J. Hebda and L. J. McCormick
Location
Samples came primarily from western North America, with a few from the Arctic and Eurasia.
Methods
We sequenced two chloroplast DNA spacer regions, trnH–psbA and trnS–G, in individuals from 199 populations and mapped haplotype distributions and their relationships using a haplotype network. We calculated genetic and molecular diversity statistics for the seven geographical regions from which we obtained samples.
Results
Fifteen haplotypes were detected, with very little divergence among them. The haplotypes are separated into two main groups by the presence or absence of a 22 bp tandem duplication. Four haplotypes are common, widespread and with substantial range overlap; 11 are rare and mostly unique to one region. Two rare haplotypes were found only in British Columbia (BC). Western North America and Asia have the highest levels of genetic and molecular diversity. Northern and southern BC have different haplotype complements.
Main conclusions
Bistorta vivipara has relatively low genetic diversity, with much less genetic structure than we expected for such a widespread species. We expected significant geographical structure due to the combined effects of genetic drift and geographical isolation. The asexual reproductive mode of B. vivipara may facilitate relatively rapid population establishment and spread compared with sexual reproduction by seed. Bistorta vivipara probably originated in Asia and spread to North America and Europe prior to the LGM. In western North America it spread to its modern distribution from Beringia and the western USA following the LGM. Populations in northern and southern BC may have different histories, possibly related to the timing and extent of glaciation. The occurrence of two unique haplotypes within BC suggests that some individuals may have survived in full glacial refugia within BC.
Geraldine A. Allen, Kendrick L. Marr, Laurie J. McCormick and Richard J. Hebda
Aim
Location
Northern Hemisphere, especially North America.
Methods
We sampled Sibbaldia from 176 localities, including 168 for S. pro-cumbens. We analysed sequence variation in three plastid DNA non-coding regions (the atpI–atpH and trnL–trnF intergenic spacers and the trnL intron), performed Bayesian phylogenetic analyses and statistical parsimony analyses on the combined sequences, and analysed the geographical patterns of haplotype distribution and genetic diversity using data from all populations.
Results
Sibbaldia procumbens probably originated in the mountains of South and East Asia. We identified highly distinct clades in Europe and North America, which overlapped on oceanic islands of the North Atlantic indicating long-distance dispersal capability. The North American clade included two lineages, one in California and the other widely distributed across the continent and North Atlantic. Haplotype diversity in the latter lineage was markedly higher to the south, suggesting mid–late Pleistocene southward displacement of North American populations with subsequent migration northwards into previously glaciated regions. In Europe, disjunct geographical regions generally harboured distinct haplotypes.
Main conclusions
Multiple Pleistocene refugia for S. procumbens occurred in both North America and Europe. North American refugia existed in California and in the southern Rocky Mountains, but in contrast with most widespread arctic–alpine species we found no evidence for a Beringian refugium. Cryptic refugia may have existed within the Cordilleran Ice Sheet. Episodes of range expansion and contraction and long-distance dispersal have all contributed to the genetic structure and widespread but fragmented distribution of this species.
Galina Gussarova, Geraldine A. Allen, Yulia Mikhaylova, Laurie J. McCormick, Virginia Mirré, Kendrick L. Marr, Richard J. Hebda and Christian Brochmann
PREMISE OF THE STUDY: Many arctic-alpine species have vast geographic ranges, but these may encompass substantial gaps whose origins are poorly understood. Here we address the phylogeographic history of Silene acaulis, a perennial cushion plant with a circumpolar distribution except for a large gap in Siberia.
METHODS: We assessed genetic variation in a range-wide sample of 103 populations using plastid DNA (pDNA) sequences and AFLPs (amplified fragment length polymorphisms). We constructed a haplotype network and performed Bayesian phylogenetic analyses based on plastid sequences. We visualized AFLP patterns using principal coordinate analysis, identified genetic groups using the program structure, and estimated genetic diversity and rarity indices by geographic region.
KEY RESULTS: The history of the main pDNA lineages was estimated to span several glaciations. AFLP data revealed a distinct division between Beringia/North America and Europe/East Greenland. These two regions shared only one of 17 pDNA haplotypes. Populations on opposite sides of the Siberian range gap (Ural Mountains and Chukotka) were genetically distinct and appear to have resulted from postglacial leading-edge colonizations. We inferred two refugia in North America (Beringia and the southern Rocky Mountains) and two in Europe (central-southern Europe and northern Europe/East Greenland). Patterns in the East Atlantic region suggested transoceanic long-distance dispersal events.
CONCLUSIONS: Silene acaulis has a highly dynamic history characterized by vicariance, regional extinction, and recolonization, with persistence in at least four refugia. Long-distance dispersal explains patterns across the Atlantic Ocean, but we found no evidence of dispersal across the Siberian range gap.
Key words: AFLP, arctic-alpine, Caryophyllaceae, disjunct distribution, phylogeography, psbD-trnT(GGU) spacer, rpL32-trnL(UAG) spacer, Silene acaulis, trnL(UAA) intron, trnL(UAA)-trnF(GAA) spacer, refugia
Kendrick L. Marr, Richard J. Hebda
We used PCA of morphological characters to confirm the presence of an undescribed Calamagrostis species in Washington and Oregon that has historically been attributed to Calamagrostis vaseyi. We propose to name this grass Calamagrostis tacomensis. It is most similar to C. foliosa although it has often been confused with C. purpurascens and C. sesquiflora all of which have similar lemma awn characteristics (i.e., the awn relatively long, exserted, and bent). Calamagrostis tacomensis has been collected at high elevations (490–2170 m) in the Washington Cascades, the Olympic Peninsula and the Steens Mountains of Oregon. The name C. vaseyi has been misapplied to our new species. The description of C. vaseyi is similar to C. rubescens. We have studied the specimen that has been attributed to be the type of C. vaseyi and it is C. purpurascens. We lectotypify C. vaseyi.
Keywords: Calamagrostis tacomensis, Calamagrostis vaseyi, Poaceae, morphology, Washington, Oregon
Kendrick L. Marr, Richard J. Hebda, and Elizabeth Anne Zamluk
The taxonomically difficult and ecologically and phytogeographically important genus, Calamagrostis, was examined for British Columbia (BC). Morphological characters were analyzed by Principal Components Analysis (PCA) to characterize taxa and to aid in the development of a new key. Eight native species (Calamagrostis canadensis, C. lapponica, C. montanensis, C. nutkaensis, C. purpurascens, C. rubescens, C. sesquiflora, and C. stricta) are confirmed to occur in British Columbia, of which C. montanensis, C. nutkaensis, C. purpurascens, C. rubescens, and C. sesquiflora are reliably distinguishable. Comparison of species distribution to regional climatic and vegetation history suggests that Calamagrostis nutkaensis and C. sesquiflora likely survived in coastal refugia during late Wisconsin glaciations. Calamagrostis purpurascens likely persisted beyond the glacial limits or within nunataks and then spread into previously glaciated sites. Two interior continental species, C. montanensis and C. rubescens, probably spread north and west from the unglaciated zone south of the Cordilleran and Laurentide ice sheets. Calamagrostis lapponica likely persisted north of the ice sheets, and then spread southward into high-elevation sites in northern and eastern BC. Calamagrostis canadensis and C. stricta probably survived south and north of the ice sheets, and then spread into the previously glaciated terrain.